Coexpression of the high molecular weight glutenin subunit 1Ax1 and puroindoline improves dough mixing properties in durum wheat (Triticum turgidum L. ssp. durum)
- PMID: 23185532
- PMCID: PMC3503773
- DOI: 10.1371/journal.pone.0050057
Coexpression of the high molecular weight glutenin subunit 1Ax1 and puroindoline improves dough mixing properties in durum wheat (Triticum turgidum L. ssp. durum)
Abstract
Wheat end-use quality mainly derives from two interrelated characteristics: the compositions of gluten proteins and grain hardness. The composition of gluten proteins determines dough rheological properties and thus confers the unique viscoelastic property on dough. One group of gluten proteins, high molecular weight glutenin subunits (HMW-GS), plays an important role in dough functional properties. On the other hand, grain hardness, which influences the milling process of flour, is controlled by Puroindoline a (Pina) and Puroindoline b (Pinb) genes. However, little is known about the combined effects of HMW-GS and PINs on dough functional properties. In this study, we crossed a Pina-expressing transgenic line with a 1Ax1-expressing line of durum wheat and screened out lines coexpressing 1Ax1 and Pina or lines expressing either 1Ax1 or Pina. Dough mixing analysis of these lines demonstrated that expression of 1Ax1 improved both dough strength and over-mixing tolerance, while expression of PINA detrimentally affected the dough resistance to extension. In lines coexpressing 1Ax1 and Pina, faster hydration of flour during mixing was observed possibly due to the lower water absorption and damaged starch caused by PINA expression. In addition, expression of 1Ax1 appeared to compensate the detrimental effect of PINA on dough resistance to extension. Consequently, coexpression of 1Ax1 and PINA in durum wheat had combined effects on dough mixing behaviors with a better dough strength and resistance to extension than those from lines expressing either 1Ax1 or Pina. The results in our study suggest that simultaneous modulation of dough strength and grain hardness in durum wheat could significantly improve its breadmaking quality and may not even impair its pastamaking potential. Therefore, coexpression of 1Ax1 and PINA in durum wheat has useful implications for breeding durum wheat with dual functionality (for pasta and bread) and may improve the economic values of durum wheat.
Conflict of interest statement
Figures

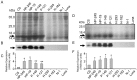
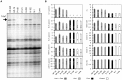
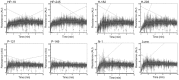
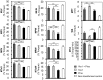
Similar articles
-
Co-expression of high-molecular-weight glutenin subunit 1Ax1 and Puroindoline a (Pina) genes in transgenic durum wheat (Triticum turgidum ssp. durum) improves milling and pasting quality.BMC Plant Biol. 2019 Apr 4;19(1):126. doi: 10.1186/s12870-019-1734-x. BMC Plant Biol. 2019. PMID: 30947699 Free PMC article.
-
The interactive effects of transgenically overexpressed 1Ax1 with various HMW-GS combinations on dough quality by introgression of exogenous subunits into an elite Chinese Wheat variety.PLoS One. 2013 Oct 22;8(10):e78451. doi: 10.1371/journal.pone.0078451. eCollection 2013. PLoS One. 2013. PMID: 24167625 Free PMC article.
-
Effect of high molecular weight glutenin subunit composition in common wheat on dough properties and steamed bread quality.J Sci Food Agric. 2014 Oct;94(13):2801-6. doi: 10.1002/jsfa.6635. Epub 2014 Mar 31. J Sci Food Agric. 2014. PMID: 24591045
-
Wheat gluten: high molecular weight glutenin subunits--structure, genetics, and relation to dough elasticity.J Food Sci. 2007 Apr;72(3):R56-63. doi: 10.1111/j.1750-3841.2007.00292.x. J Food Sci. 2007. PMID: 17995810 Review.
-
Increasing the Versatility of Durum Wheat through Modifications of Protein and Starch Composition and Grain Hardness.Foods. 2022 May 24;11(11):1532. doi: 10.3390/foods11111532. Foods. 2022. PMID: 35681282 Free PMC article. Review.
Cited by
-
Genomics-Enabled Analysis of Puroindoline b2 Genes Identifies New Alleles in Wheat and Related Triticeae Species.Int J Mol Sci. 2020 Feb 14;21(4):1304. doi: 10.3390/ijms21041304. Int J Mol Sci. 2020. PMID: 32075191 Free PMC article.
-
Genetic Modification for Wheat Improvement: From Transgenesis to Genome Editing.Biomed Res Int. 2019 Mar 10;2019:6216304. doi: 10.1155/2019/6216304. eCollection 2019. Biomed Res Int. 2019. PMID: 30956982 Free PMC article. Review.
-
Toward the Genetic Basis and Multiple QTLs of Kernel Hardness in Wheat.Plants (Basel). 2020 Nov 24;9(12):1631. doi: 10.3390/plants9121631. Plants (Basel). 2020. PMID: 33255282 Free PMC article.
-
Co-expression of high-molecular-weight glutenin subunit 1Ax1 and Puroindoline a (Pina) genes in transgenic durum wheat (Triticum turgidum ssp. durum) improves milling and pasting quality.BMC Plant Biol. 2019 Apr 4;19(1):126. doi: 10.1186/s12870-019-1734-x. BMC Plant Biol. 2019. PMID: 30947699 Free PMC article.
-
Prospecting for Microelement Function and Biosafety Assessment of Transgenic Cereal Plants.Front Plant Sci. 2018 Mar 15;9:326. doi: 10.3389/fpls.2018.00326. eCollection 2018. Front Plant Sci. 2018. PMID: 29599791 Free PMC article. Review.
References
-
- Shewry PR (2009) Wheat. J Exp Bot 60: 1537–1553. - PubMed
-
- Bushuk W (1998) Wheat breeding for end-product use. Euphytica 100: 137–145.
-
- Leon E, Marin S, Gimenez MJ, Piston F, Rodriguez-Quijano M, et al. (2009) Mixing properties and dough functionality of transgenic lines of a commercial wheat cultivar expressing the 1Ax1, 1Dx5 and 1Dy10 HMW glutenin subunit genes. J Cereal Sci 49: 148–156.
-
- Gazza L, Sgrulletta D, Cammerata A, Gazzelloni G, Perenzin M, et al. (2011) Pastamaking and breadmaking quality of soft-textured durum wheat lines. J Cereal Sci 54: 481–487.
-
- Altpeter F, Vasil V, Srivastava V, Vasil IK (1996) Integration and expression of the high molecular weight glutenin subunit 1Ax1 gene into wheat. Nat Biotechnol 14: 1155–1159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

