Multiple-clone activation of hypnozoites is the leading cause of relapse in Plasmodium vivax infection
- PMID: 23185469
- PMCID: PMC3503861
- DOI: 10.1371/journal.pone.0049871
Multiple-clone activation of hypnozoites is the leading cause of relapse in Plasmodium vivax infection
Abstract
Background: Plasmodium vivax infection is characterized by a dormant hepatic stage, the hypnozoite that is activated at varying periods of time after clearance of the primary acute blood-stage, resulting in relapse. Differentiation between treatment failure and new infections requires characterization of initial infections, relapses, and clone multiplicity in vivax malaria infections.
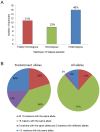
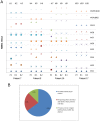
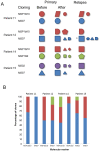
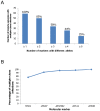
Methodology/principal findings: Parasite DNA obtained from primary/relapse paired blood samples of 30 patients with P. vivax infection in Brazil was analyzed using 10 molecular markers (8 microsatellites and MSP-1 blocks 2 and 10). Cloning of PCR products and genotyping was used to identify low-frequency clones of parasites. We demonstrated a high frequency of multiple-clone infections in both primary and relapse infections. Few alleles were identified per locus, but the combination of these alleles produced many haplotypes. Consequently, the majority of parasites involved in relapse showed haplotypes that were distinct from those of primary infections. Plasmodium vivax relapse was characterized by temporal variations in the predominant parasite clones.
Conclusions/significance: The high rate of low frequency alleles observed in both primary and relapse infections, along with temporal variation in the predominant alleles, might be the source of reported heterologous hypnozoite activation. Our findings complicate the concept of heterologous activation, suggesting the involvement of undetermined mechanisms based on host or environmental factors in the simultaneous activation of multiple clones of hypnozoites.
Conflict of interest statement
Figures
Similar articles
-
Using Amplicon Deep Sequencing to Detect Genetic Signatures of Plasmodium vivax Relapse.J Infect Dis. 2015 Sep 15;212(6):999-1008. doi: 10.1093/infdis/jiv142. Epub 2015 Mar 6. J Infect Dis. 2015. PMID: 25748326 Free PMC article.
-
Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites.J Infect Dis. 2007 Apr 1;195(7):927-33. doi: 10.1086/512241. Epub 2007 Feb 26. J Infect Dis. 2007. PMID: 17330781 Clinical Trial.
-
Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals.J Infect Dis. 2007 Apr 1;195(7):934-41. doi: 10.1086/512242. Epub 2007 Feb 26. J Infect Dis. 2007. PMID: 17330782
-
Plasmodium vivax Latent Liver Stage Infection and Relapse: Biological Insights and New Experimental Tools.Annu Rev Microbiol. 2021 Oct 8;75:87-106. doi: 10.1146/annurev-micro-032421-061155. Epub 2021 Jul 1. Annu Rev Microbiol. 2021. PMID: 34196569 Review.
-
A closer look at multiple-clone Plasmodium vivax infections: detection methods, prevalence and consequences.Mem Inst Oswaldo Cruz. 2009 Feb;104(1):67-73. doi: 10.1590/s0074-02762009000100011. Mem Inst Oswaldo Cruz. 2009. PMID: 19274379 Review.
Cited by
-
Association between CYP2D6 phenotype and recurrence of Plasmodium vivax infection in south Korean patients.Malar J. 2022 Oct 10;21(1):289. doi: 10.1186/s12936-022-04311-6. Malar J. 2022. PMID: 36217154 Free PMC article.
-
Delayed gametocyte clearance in Plasmodium vivax malaria is associated with polymorphisms in the cytochrome P450 reductase (CPR).Antimicrob Agents Chemother. 2024 Apr 3;68(4):e0120423. doi: 10.1128/aac.01204-23. Epub 2024 Feb 27. Antimicrob Agents Chemother. 2024. PMID: 38411047 Free PMC article.
-
Plasmodium vivax isolates from Cambodia and Thailand show high genetic complexity and distinct patterns of P. vivax multidrug resistance gene 1 (pvmdr1) polymorphisms.Am J Trop Med Hyg. 2013 Jun;88(6):1116-23. doi: 10.4269/ajtmh.12-0701. Epub 2013 Mar 18. Am J Trop Med Hyg. 2013. PMID: 23509126 Free PMC article.
-
Activation of minority-variant Plasmodium vivax hypnozoites following artesunate + amodiaquine treatment in a 23-year old man with relapsing malaria in Antananarivo, Madagascar.Malar J. 2013 May 31;12:177. doi: 10.1186/1475-2875-12-177. Malar J. 2013. PMID: 23721298 Free PMC article.
-
Plasmodium vivax Landscape in Brazil: Scenario and Challenges.Am J Trop Med Hyg. 2016 Dec 28;95(6 Suppl):87-96. doi: 10.4269/ajtmh.16-0204. Epub 2016 Oct 5. Am J Trop Med Hyg. 2016. PMID: 27708190 Free PMC article.
References
-
- World Health Organization (2010) World Malaria Report 2010. Geneva: World Health Organization.
-
- Ministério da Saude, Secretaria de Vigilancia em Saude, Departamento de Vigilancia Epidemiologic. (2010) Aspectos epidemiológicos da Malária. Brasilia: Ministerio da Saude.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

