Drosophila patterning is established by differential association of mRNAs with P bodies
- PMID: 23178881
- PMCID: PMC4066581
- DOI: 10.1038/ncb2627
Drosophila patterning is established by differential association of mRNAs with P bodies
Abstract
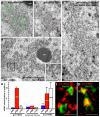
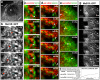
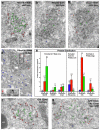
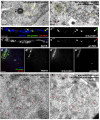
The primary embryonic axes in flies, frogs and fish are formed through translational regulation of localized transcripts before fertilization. In Drosophila melanogaster, the axes are established through the transport and translational regulation of gurken (grk) and bicoid (bcd) messenger RNA in the oocyte and embryo. Both transcripts are translationally silent while being localized within the oocyte along microtubules by cytoplasmic dynein. Once localized, grk is translated at the dorsoanterior of the oocyte to send a TGF-α signal to the overlying somatic cells. In contrast, bcd is translationally repressed in the oocyte until its activation in early embryos when it forms an anteroposterior morphogenetic gradient. How this differential translational regulation is achieved is not fully understood. Here, we address this question using ultrastructural analysis, super-resolution microscopy and live-cell imaging. We show that grk and bcd ribonucleoprotein (RNP) complexes associate with electron-dense bodies that lack ribosomes and contain translational repressors. These properties are characteristic of processing bodies (P bodies), which are considered to be regions of cytoplasm where decisions are made on the translation and degradation of mRNA. Endogenous grk mRNA forms dynamic RNP particles that become docked and translated at the periphery of P bodies, where we show that the translational activator Oo18 RNA-binding protein (Orb, a homologue of CEPB) and the anchoring factor Squid (Sqd) are also enriched. In contrast, an excess of grk mRNA becomes localized inside the P bodies, where endogenous bcd mRNA is localized and translationally repressed. Interestingly, bcd mRNA dissociates from P bodies in embryos following egg activation, when it is known to become translationally active. We propose a general principle of translational regulation during axis specification involving remodelling of transport RNPs and dynamic partitioning of different transcripts between the translationally active edge of P bodies and their silent core.
Figures
Similar articles
-
Localized Translation of gurken/TGF-α mRNA during Axis Specification Is Controlled by Access to Orb/CPEB on Processing Bodies.Cell Rep. 2016 Mar 15;14(10):2451-62. doi: 10.1016/j.celrep.2016.02.038. Epub 2016 Mar 3. Cell Rep. 2016. PMID: 26947065 Free PMC article.
-
Repression of Gurken translation by a meiotic checkpoint in Drosophila oogenesis is suppressed by a reduction in the dose of eIF1A.Development. 2014 Oct;141(20):3910-21. doi: 10.1242/dev.109306. Epub 2014 Sep 17. Development. 2014. PMID: 25231760 Free PMC article.
-
Direct observation of regulated ribonucleoprotein transport across the nurse cell/oocyte boundary.Mol Biol Cell. 2007 Jun;18(6):2254-63. doi: 10.1091/mbc.e06-10-0959. Epub 2007 Apr 11. Mol Biol Cell. 2007. PMID: 17429069 Free PMC article.
-
[Source of asymmetry in ontogeny: early polarization of the germline cyst and oocyte in Drosophila].Genetika. 2008 Sep;44(9):1157-71. Genetika. 2008. PMID: 18846812 Review. Russian.
-
Localization, anchoring and translational control of oskar, gurken, bicoid and nanos mRNA during Drosophila oogenesis.Fly (Austin). 2009 Jan-Mar;3(1):15-28. doi: 10.4161/fly.3.1.7751. Epub 2009 Jan 2. Fly (Austin). 2009. PMID: 19182536 Review.
Cited by
-
Testing Models of mRNA Localization Reveals Robustness Regulated by Reducing Transport between Cells.Biophys J. 2019 Dec 3;117(11):2154-2165. doi: 10.1016/j.bpj.2019.10.025. Epub 2019 Oct 24. Biophys J. 2019. PMID: 31708163 Free PMC article.
-
Germ granules in development.Development. 2023 Jan 15;150(2):dev201037. doi: 10.1242/dev.201037. Epub 2023 Jan 30. Development. 2023. PMID: 36715566 Free PMC article.
-
The mRNA dynamics underpinning translational control mechanisms of Drosophila melanogaster oogenesis.Biochem Soc Trans. 2024 Oct 30;52(5):2087-2099. doi: 10.1042/BST20231293. Biochem Soc Trans. 2024. PMID: 39263986 Free PMC article. Review.
-
The temporally controlled expression of Drongo, the fruit fly homolog of AGFG1, is achieved in female germline cells via P-bodies and its localization requires functional Rab11.RNA Biol. 2016 Nov;13(11):1117-1132. doi: 10.1080/15476286.2016.1218592. Epub 2016 Aug 11. RNA Biol. 2016. PMID: 27654348 Free PMC article.
-
The molecular mechanisms underpinning maternal mRNA dormancy.Biochem Soc Trans. 2024 Apr 24;52(2):861-871. doi: 10.1042/BST20231122. Biochem Soc Trans. 2024. PMID: 38477334 Free PMC article. Review.
References
-
- St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat. Rev. Mol. Cell Biol. 2005;6:363–375. - PubMed
-
- Weil TT, Forrest KM, Gavis ER. Localization of bicoid mRNA in late oocytes is maintained by continual active transport. Dev Cell. 2006;11:251–262. doi:S1534-5807(06)00262-0 [pii]10.1016/j.devcel.2006.06.006. - PubMed
-
- Cha BJ, Koppetsch BS, Theurkauf WE. In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell. 2001;106:35–46. doi:S0092-8674(01)00419-6 [pii] - PubMed
-
- Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGFa-like protein. Cell. 1993;75:165–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

