Host cell factors as antiviral targets in arenavirus infection
- PMID: 23170173
- PMCID: PMC3499820
- DOI: 10.3390/v4091569
Host cell factors as antiviral targets in arenavirus infection
Abstract
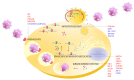
Among the members of the Arenaviridae family, Lassa virus and Junin virus generate periodic annual outbreaks of severe human hemorrhagic fever (HF) in endemic areas of West Africa and Argentina, respectively. Given the human health threat that arenaviruses represent and the lack of a specific and safe chemotherapy, the search for effective antiviral compounds is a continuous demanding effort. Since diverse host cell pathways and enzymes are used by RNA viruses to fulfill their replicative cycle, the targeting of a host process has turned an attractive antiviral approach in the last years for many unrelated virus types. This strategy has the additional benefit to reduce the serious challenge for therapy of RNA viruses to escape from drug effects through selection of resistant variants triggered by their high mutation rate. This article focuses on novel strategies to identify inhibitors for arenavirus therapy, analyzing the potential for antiviral developments of diverse host factors essential for virus infection.
Keywords: Junin virus; antiviral; arenavirus; hemorrhagic fever; host cell target.
Figures
Similar articles
-
Determining the Virus Life-Cycle Stage Blocked by an Antiviral.Methods Mol Biol. 2018;1604:371-392. doi: 10.1007/978-1-4939-6981-4_28. Methods Mol Biol. 2018. PMID: 28986849
-
Research efforts to control highly pathogenic arenaviruses: a summary of the progress and gaps.J Clin Virol. 2015 Mar;64:120-7. doi: 10.1016/j.jcv.2014.12.004. Epub 2014 Dec 18. J Clin Virol. 2015. PMID: 25549822 Review.
-
Identification of Clotrimazole Derivatives as Specific Inhibitors of Arenavirus Fusion.J Virol. 2019 Mar 5;93(6):e01744-18. doi: 10.1128/JVI.01744-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626681 Free PMC article.
-
[Arenavirus research and antiviral candidate].Uirusu. 2018;68(1):51-62. doi: 10.2222/jsv.68.51. Uirusu. 2018. PMID: 31105135 Review. Japanese.
-
The role of the vascular endothelium in arenavirus haemorrhagic fevers.Thromb Haemost. 2009 Dec;102(6):1024-9. doi: 10.1160/TH09-06-0357. Thromb Haemost. 2009. PMID: 19967131 Review.
Cited by
-
Small-molecule probes targeting the viral PPxY-host Nedd4 interface block egress of a broad range of RNA viruses.J Virol. 2014 Jul;88(13):7294-306. doi: 10.1128/JVI.00591-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741084 Free PMC article.
-
A Proteomics Survey of Junín Virus Interactions with Human Proteins Reveals Host Factors Required for Arenavirus Replication.J Virol. 2018 Jan 30;92(4):e01565-17. doi: 10.1128/JVI.01565-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29187543 Free PMC article.
-
Effects of the Natural Flavonoid Quercetin on Arenavirus Junín Infection.Viruses. 2023 Aug 15;15(8):1741. doi: 10.3390/v15081741. Viruses. 2023. PMID: 37632083 Free PMC article.
-
Role of the ERK1/2 Signaling Pathway in the Replication of Junín and Tacaribe Viruses.Viruses. 2018 Apr 17;10(4):199. doi: 10.3390/v10040199. Viruses. 2018. PMID: 29673133 Free PMC article.
-
A host-oriented inhibitor of Junin Argentine hemorrhagic fever virus egress.J Virol. 2014 May;88(9):4736-43. doi: 10.1128/JVI.03757-13. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522922 Free PMC article.
References
-
- McCormick J.B., Fisher-Hoch S.P. Lassa fever. Curr. Top. Microbiol. Immunol. 2002;262:75–109. - PubMed
-
- Fisher S.A., Graham M.B., Kuehnert M.J., Kotton C.N., Srinivasan A., Marty F.M., Comer J.A., Guarner J., Paddock C.D., DeMeo D.L., et al. LCMV in Transplant recipients investigation team. Transmission of lymphocytic choriomeningiris virus by organ transplantation. N. Engl. J. Med. 2006;354:2235–2249. doi: 10.1056/NEJMoa053240. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

