The Sec1/Munc18 protein Vps45 regulates cellular levels of its SNARE binding partners Tlg2 and Snc2 in Saccharomyces cerevisiae
- PMID: 23166732
- PMCID: PMC3498219
- DOI: 10.1371/journal.pone.0049628
The Sec1/Munc18 protein Vps45 regulates cellular levels of its SNARE binding partners Tlg2 and Snc2 in Saccharomyces cerevisiae
Abstract
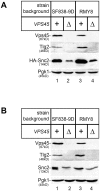
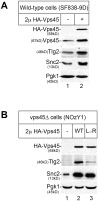
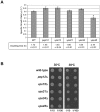
Intracellular membrane trafficking pathways must be tightly regulated to ensure proper functioning of all eukaryotic cells. Central to membrane trafficking is the formation of specific SNARE (soluble N-ethylmeleimide-sensitive factor attachment protein receptor) complexes between proteins on opposing lipid bilayers. The Sec1/Munc18 (SM) family of proteins play an essential role in SNARE-mediated membrane fusion, and like the SNAREs are conserved through evolution from yeast to humans. The SM protein Vps45 is required for the formation of yeast endosomal SNARE complexes and is thus essential for traffic through the endosomal system. Here we report that, in addition to its role in regulating SNARE complex assembly, Vps45 regulates cellular levels of its SNARE binding partners: the syntaxin Tlg2 and the v-SNARE Snc2: Cells lacking Vps45 have reduced cellular levels of Tlg2 and Snc2; and elevation of Vps45 levels results in concomitant increases in the levels of both Tlg2 and Snc2. As well as regulating traffic through the endosomal system, the Snc v-SNAREs are also required for exocytosis. Unlike most vps mutants, cells lacking Vps45 display multiple growth phenotypes. Here we report that these can be reversed by selectively restoring Snc2 levels in vps45 mutant cells. Our data indicate that as well as functioning as part of the machinery that controls SNARE complex assembly, Vps45 also plays a key role in determining the levels of its cognate SNARE proteins; another key factor in regulation of membrane traffic.
Conflict of interest statement
Figures

Similar articles
-
The Sec1/Munc18 protein Vps45 holds the Qa-SNARE Tlg2 in an open conformation.Elife. 2020 Aug 17;9:e60724. doi: 10.7554/eLife.60724. Elife. 2020. PMID: 32804076 Free PMC article.
-
A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly.Science. 2015 Sep 4;349(6252):1111-4. doi: 10.1126/science.aac7906. Science. 2015. PMID: 26339030 Free PMC article.
-
Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex.Mol Biol Cell. 2012 Dec;23(23):4611-22. doi: 10.1091/mbc.E12-05-0343. Epub 2012 Oct 10. Mol Biol Cell. 2012. PMID: 23051737 Free PMC article.
-
Membrane fusion: grappling with SNARE and SM proteins.Science. 2009 Jan 23;323(5913):474-7. doi: 10.1126/science.1161748. Science. 2009. PMID: 19164740 Free PMC article. Review.
-
At the junction of SNARE and SM protein function.Curr Opin Cell Biol. 2010 Aug;22(4):488-95. doi: 10.1016/j.ceb.2010.04.006. Epub 2010 May 12. Curr Opin Cell Biol. 2010. PMID: 20471239 Free PMC article. Review.
Cited by
-
Components of the SNARE-containing regulon are co-regulated in root cells undergoing defense.Plant Signal Behav. 2017 Feb;12(2):e1274481. doi: 10.1080/15592324.2016.1274481. Plant Signal Behav. 2017. PMID: 28010187 Free PMC article.
-
Study on the differential proteomics of rat hippocampal mitochondria during deep hypothermic circulatory arrest.Ann Transl Med. 2021 Feb;9(4):346. doi: 10.21037/atm-21-95. Ann Transl Med. 2021. PMID: 33708973 Free PMC article.
-
A congenital neutrophil defect syndrome associated with mutations in VPS45.N Engl J Med. 2013 Jul 4;369(1):54-65. doi: 10.1056/NEJMoa1301296. Epub 2013 Jun 5. N Engl J Med. 2013. PMID: 23738510 Free PMC article.
-
Phosphorylation of the N-terminus of Syntaxin-16 controls interaction with mVps45 and GLUT4 trafficking in adipocytes.PeerJ. 2023 Jul 24;11:e15630. doi: 10.7717/peerj.15630. eCollection 2023. PeerJ. 2023. PMID: 37520260 Free PMC article.
-
SNARE Protein Snc1 Is Essential for Vesicle Trafficking, Membrane Fusion and Protein Secretion in Fungi.Cells. 2023 Jun 5;12(11):1547. doi: 10.3390/cells12111547. Cells. 2023. PMID: 37296667 Free PMC article. Review.
References
-
- Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116: 153–166. - PubMed
-
- Jahn R, Scheller RH (2006) SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol 7: 631–643. - PubMed
-
- Ungar D, Hughson FM (2003) Snare protein structure and function. Annu Rev Cell Dev Biol 19: 493–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

