Ataxia telangiectasia mutated-dependent regulation of topoisomerase II alpha expression and sensitivity to topoisomerase II inhibitor
- PMID: 23163762
- PMCID: PMC7657118
- DOI: 10.1111/cas.12067
Ataxia telangiectasia mutated-dependent regulation of topoisomerase II alpha expression and sensitivity to topoisomerase II inhibitor
Abstract
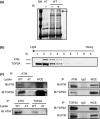
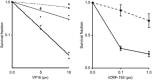
Topoisomerase II alpha (TOP2A) has a crucial role in proper chromosome condensation and segregation. Here we report the interaction of TOP2A with ataxia telangiectasia mutated (ATM) and its phosphorylation in an ATM-dependent manner after DNA damage. In vitro kinase assay and site-directed mutagenesis studies revealed that serine 1512 is the target of phosphorylation through ATM. Serine 1512 to Alanine mutation of TOP2A showed increased stability of the protein, retaining TOP2A activity at least with regard to cell survival activity. Ataxia telangiectasia-derived cell lines showed high levels of TOP2A that were associated with hypersensitivity to the TOP2 inhibitor etoposide. These findings suggest that ATM-dependent TOP2A modification is required for proper regulation of TOP2 stability and subsequently of the sensitivity to TOP2 inhibitor. In a lymphoblastoid cell line derived from a patient who developed MLL rearrangement, positive infant leukemia, defective ATM expression, and increased TOP2A expression were shown. It was intriguing that hypersensitivity to TOP2 inhibitor and susceptibility to MLL gene rearrangement were shown by low-dose etoposide exposure in this cell line. Thus, our findings have clinically important implications for the pathogenesis of infantile acute leukemia as well as treatment-associated secondary leukemia following exposure to TOP2 inhibitors.
© 2012 Japanese Cancer Association.
Figures



Similar articles
-
The catalytic topoisomerase II inhibitor dexrazoxane induces DNA breaks, ATF3 and the DNA damage response in cancer cells.Br J Pharmacol. 2015 May;172(9):2246-57. doi: 10.1111/bph.13046. Epub 2015 Feb 27. Br J Pharmacol. 2015. PMID: 25521189 Free PMC article.
-
Cellular processing pathways contribute to the activation of etoposide-induced DNA damage responses.DNA Repair (Amst). 2008 Mar 1;7(3):452-63. doi: 10.1016/j.dnarep.2007.12.002. DNA Repair (Amst). 2008. PMID: 18206427
-
Phosphorylation of p53 on Ser15 during cell cycle caused by Topo I and Topo II inhibitors in relation to ATM and Chk2 activation.Cell Cycle. 2008 Oct;7(19):3048-55. doi: 10.4161/cc.7.19.6750. Epub 2008 Oct 6. Cell Cycle. 2008. PMID: 18802408 Free PMC article.
-
ATM and the molecular pathogenesis of ataxia telangiectasia.Annu Rev Pathol. 2012;7:303-21. doi: 10.1146/annurev-pathol-011811-132509. Epub 2011 Oct 24. Annu Rev Pathol. 2012. PMID: 22035194 Review.
-
XCIND as a genetic disease of X-irradiation hypersensitivity and cancer susceptibility.Int J Hematol. 2013 Jan;97(1):37-42. doi: 10.1007/s12185-012-1240-5. Epub 2012 Dec 25. Int J Hematol. 2013. PMID: 23266960 Review.
Cited by
-
Leukemogenic rearrangements at the mixed lineage leukemia gene (MLL)-multiple rather than a single mechanism.Front Cell Dev Biol. 2015 Jun 25;3:41. doi: 10.3389/fcell.2015.00041. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26161385 Free PMC article. Review.
-
Topo2A as a prognostic biomarker for patients with resectable esophageal squamous cell carcinomas.Med Oncol. 2015 Jan;32(1):396. doi: 10.1007/s12032-014-0396-7. Epub 2014 Nov 29. Med Oncol. 2015. PMID: 25432700
-
Dysregulation of the DNA Damage Response and KMT2A Rearrangement in Fetal Liver Hematopoietic Cells.PLoS One. 2015 Dec 11;10(12):e0144540. doi: 10.1371/journal.pone.0144540. eCollection 2015. PLoS One. 2015. PMID: 26657054 Free PMC article.
-
Cellular functions of the protein kinase ATM and their relevance to human disease.Nat Rev Mol Cell Biol. 2021 Dec;22(12):796-814. doi: 10.1038/s41580-021-00394-2. Epub 2021 Aug 24. Nat Rev Mol Cell Biol. 2021. PMID: 34429537 Review.
-
Systematic polypharmacology and drug repurposing via an integrated L1000-based Connectivity Map database mining.R Soc Open Sci. 2018 Nov 28;5(11):181321. doi: 10.1098/rsos.181321. eCollection 2018 Nov. R Soc Open Sci. 2018. PMID: 30564416 Free PMC article.
References
-
- Athma P, Rappaport R, Swift M. Molecular genotyping shows that ataxia‐telangiectasia heterozygotes are predisposed to breast cancer. Cancer Genet Cytogenet 1996; 92: 130–4. - PubMed
-
- Shiloh Y. ATM and ATR: networking cellular responses to DNA damage. Curr Opin Genet Dev 2001; 11: 71–7. - PubMed
-
- Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol 2000; 1: 179–86. - PubMed
-
- Isaacs RJ, Davies SL, Sandri MI, Redwood C, Wells NJ, Hickson ID. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta 1998; 1400: 121–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

