Blockade of inhibitors of apoptosis (IAPs) in combination with tumor-targeted delivery of tumor necrosis factor-α leads to synergistic antitumor activity
- PMID: 23154431
- PMCID: PMC3534156
- DOI: 10.1038/cgt.2012.83
Blockade of inhibitors of apoptosis (IAPs) in combination with tumor-targeted delivery of tumor necrosis factor-α leads to synergistic antitumor activity
Abstract
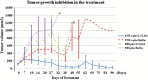
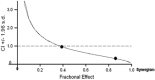
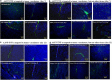
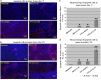
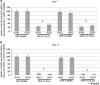
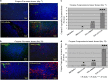
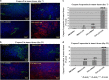
In the current study, we examined whether the combination of tumor vasculature-targeted gene therapy with adeno-associated virus bacteriophage-tumor necrosis factor-α (AAVP-TNF-α) and/or the orally administered LCL161, an antagonist of inhibitors of apoptosis proteins (IAPs), enhanced antitumor efficacy without systemic toxicity. M21 human melanoma xenografts were grown subcutaneously in nude mice. Mice were treated according to one of four treatment regimens: AAVP-TNF-α alone (AAVP-TNF-α plus sodium acetate-acetic acid (NaAc) buffer) via tail vein injection; LCL161 alone (phosphate-buffered saline (PBS) plus LCL161) via oral gavage; AAVP-TNF-α plus LCL161; and PBS plus NaAc Buffer as a control group. Tumor volume, survival and toxicity were analyzed. AAVP trafficking and TNF-α production in vivo were detected on days 7 and 21 by real-time PCR, enzyme-linked immunosorbent assay and immunofluorescence. The levels of apoptosis and activation of caspases were assessed on days 7 and 21 by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) and immunofluorescence assays. Our results showed that the combination of AAVP-TNF-α and LCL161 significantly inhibited tumor growth and prolonged survival in mice with melanoma xenografts. The combination of AAVP-TNF-α and LCL161 was also significantly more effective than either agent alone, showing a synergistic effect without systemic toxicity.
Figures



Similar articles
-
Tumor vasculature-targeted delivery of tumor necrosis factor-alpha.Cancer. 2009 Jan 1;115(1):128-39. doi: 10.1002/cncr.24001. Cancer. 2009. PMID: 19090007 Free PMC article.
-
LCL161 increases paclitaxel-induced apoptosis by degrading cIAP1 and cIAP2 in NSCLC.J Exp Clin Cancer Res. 2016 Sep 30;35(1):158. doi: 10.1186/s13046-016-0435-7. J Exp Clin Cancer Res. 2016. PMID: 27737687 Free PMC article.
-
Targeted AAVP-based therapy in a mouse model of human glioblastoma: a comparison of cytotoxic versus suicide gene delivery strategies.Cancer Gene Ther. 2020 May;27(5):301-310. doi: 10.1038/s41417-019-0101-2. Epub 2019 May 27. Cancer Gene Ther. 2020. PMID: 31130731 Free PMC article.
-
The novel SMAC mimetic birinapant exhibits potent activity against human melanoma cells.Clin Cancer Res. 2013 Apr 1;19(7):1784-94. doi: 10.1158/1078-0432.CCR-12-2518. Epub 2013 Feb 12. Clin Cancer Res. 2013. PMID: 23403634 Free PMC article.
-
Tumor necrosis factor as an antineoplastic agent: pitfalls and promises.Cell Mol Life Sci. 1998 Dec;54(12):1291-8. doi: 10.1007/s000180050255. Cell Mol Life Sci. 1998. PMID: 9893706 Free PMC article. Review.
Cited by
-
Best of most possible worlds: Hybrid gene therapy vectors based on parvoviruses and heterologous viruses.Mol Ther. 2021 Dec 1;29(12):3359-3382. doi: 10.1016/j.ymthe.2021.04.005. Epub 2021 Apr 5. Mol Ther. 2021. PMID: 33831556 Free PMC article. Review.
-
Initial Steps for the Development of a Phage-Mediated Gene Replacement Therapy Using CRISPR-Cas9 Technology.J Clin Med. 2020 May 16;9(5):1498. doi: 10.3390/jcm9051498. J Clin Med. 2020. PMID: 32429407 Free PMC article.
-
SMAC Mimetics for the Treatment of Lung Carcinoma: Present Development and Future Prospects.Mini Rev Med Chem. 2024;24(14):1334-1352. doi: 10.2174/0113895575269644231120104501. Mini Rev Med Chem. 2024. PMID: 38275029 Review.
-
Inhibitor of Apoptosis Proteins (IAPs) are commonly dysregulated in GIST and can be pharmacologically targeted to enhance the pro-apoptotic activity of imatinib.Oncotarget. 2016 Jul 5;7(27):41390-41403. doi: 10.18632/oncotarget.9159. Oncotarget. 2016. PMID: 27167336 Free PMC article.
-
Preparation and characterization of novel chitosan-protamine nanoparticles for nucleus-targeted anticancer drug delivery.Int J Nanomedicine. 2016 Nov 14;11:6035-6046. doi: 10.2147/IJN.S117066. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27881917 Free PMC article.
References
-
- Larocca D, Kassner PD, Witte A, Ladner RC, Pierce GF, Baird A. Gene transfer to mammalian cells using genetically targeted filamentous bacteriophage. FASEB J. 1999;13:727–734. - PubMed
-
- Barrow PA, Soothill JS. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 1997;5:268–271. - PubMed
-
- Ivanenkov V, Felici F, Menon AG. Uptake and intracellular fate of phage display vectors in mammalian cells. Biochim Biophys Acta. 1999;1448:450–462. - PubMed
-
- Weber-Dabrowska B, Mulczyk M, Gorski A. Bacteriophages as an efficient therapy for antibiotic-resistant septicemia in man. Transplant Proc. 2003;35:1385–1386. - PubMed
-
- Borysowski J, Gorski A. Is phage therapy acceptable in the immunocompromised host. Int J Infect Dis. 2008;12:466–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

