Febuxostat for treating chronic gout
- PMID: 23152264
- PMCID: PMC4058893
- DOI: 10.1002/14651858.CD008653.pub2
Febuxostat for treating chronic gout
Abstract
Background: Gout is the most common inflammatory arthritis in men over 40 years and has an increasing prevalence among postmenopausal women. Lowering serum uric acid levels remains one of the primary goals in the treatment of chronic gout. In clinical trials, febuxostat has been shown to be effective in lowering serum uric acid levels to < 6.0 mg/dL.
Objectives: To evaluate the benefits and harms of febuxostat for chronic gout.
Search methods: We searched The Cochrane Library, MEDLINE, EMBASE, and International Pharmaceutical Abstracts from inception to July 2011. The ClinicalTrials.gov website was searched for references to trials of febuxostat. Our search did not include any restrictions.
Selection criteria: Two authors independently reviewed the search results and disagreements were resolved by discussion. We included any controlled clinical trial or open label trial (OLT) using febuxostat at any dose.
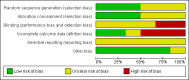
Data collection and analysis: Data and risk of bias were independently extracted by two authors and summarised in a meta-analysis. Continuous data were expressed as mean difference and dichotomous data as risk ratio (RR).
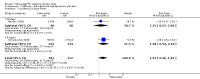
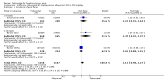
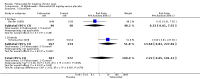
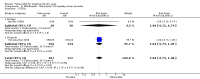
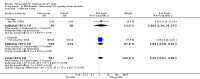
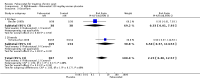
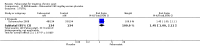
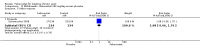
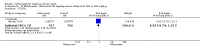
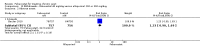
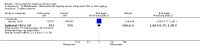
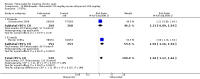
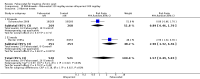
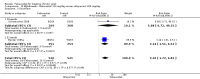
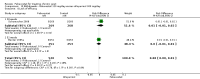
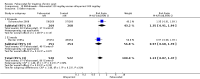
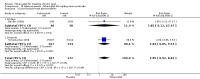
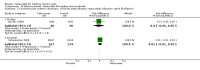
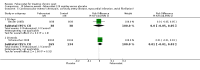
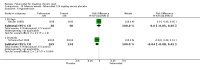
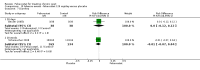
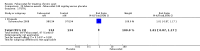
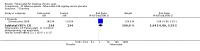
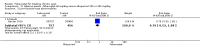
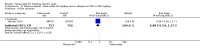
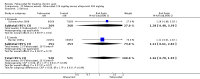
Main results: Four randomised trials and two OLTs with 3978 patients were included. Risk of bias differed by outcome, ranging from low to high risk of bias. Included studies failed to report on five to six of the nine outcome measures recommended by OMERACT. Patients taking febuxostat 120 mg and 240 mg reported more frequent gout flares than in the placebo group at 4 to 28 weeks (RR 1.7; 95% CI 1.3 to 2.3, and RR 2.6; 95% CI 1.8 to 3.7 respectively). No statistically significant differences were observed at 40 mg and 80 mg. Compared to placebo, patients on febuxostat 40 mg were 40.1 times more likely to achieve serum uric acid levels < 6.0 mg/dL at 4 weeks (95% CI 2.5 to 639), with an absolute treatment benefit of 56% (95% CI 37% to 71%). For febuxostat 80 mg and 120 mg, patients were 68.9 and 80.7 times more likely to achieve serum uric acid levels < 6.0 mg/dL at their final visit compared to placebo (95% CI 13.8 to 343.9, 95% CI 16.0 to 405.5), respectively; with an absolute treatment benefit of 75% and 87% (95% CI 68 to 80% and 81 to 91%), respectively. Total discontinuation rates were significantly higher in the febuxostat 80 mg group compared to placebo (RR 1.4; 95% CI 1.0 to 2.0, absolute risk increase 11%; 95% CI 3 to 19%). No other differences were observed.When comparing allopurinol to febuxostat at 24 to 52 weeks, the number of gout flares was not significantly different between the two groups, except for febuxostat 240 mg (RR 2.3; 95% CI 1.7 to 3.0). Patients on febuxostat 40 mg showed no statistically significant differences in benefits or harms. Patients on febuxostat 80 mg and 120 mg were 1.8 and 2.2 times more likely to achieve serum uric acid levels < 6.0 mg/dL at their final visit (95% CI 1.6 to 2.2, 95% CI 1.9 to 2.5) with an absolute treatment benefit of 29% and 44% (95% CI 25% to 33%, 95% CI 38% to 50%), respectively, at 24 to 52 weeks. Total discontinuation rates were higher for febuxostat 80 mg and 120 mg compared to allopurinol (RR 1.5; 95% CI 1.2 to 1.8, absolute risk increase 11%; 95% CI 6% to 16%; and RR 2.6; 95% CI 2.0 to 3.3, absolute risk increase 20%; 95% CI 3% to 14%, respectively). Discontinuations due to adverse events were similar across groups. Total adverse events were lower for febuxostat 80 mg and 120 mg compared with allopurinol (RR 0.93; 95% CI 0.87 to 0.99, absolute risk increase 6%; 95% CI 0.7% to 11%; and RR 0.90; 95% CI 0.84 to 0.96, absolute risk increase 8%; 95% CI 3% to 13%, respectively). No other relevant differences were noted.After 3 years of follow-up there were no statistically significant differences regarding effectiveness and harms between febuxostat 80 mg or 120 mg and allopurinol groups (adverse event rate per 100 patient-years 227, 216, and 246, respectively).
Authors' conclusions: Although the incidence of gout flares requiring treatment may be increased in patients taking febuxostat compared to placebo or allopurinol during early treatment, no such increase in gout flares was observed in the long-term follow-up study when compared to allopurinol. Febuxostat at any dose was shown to be beneficial in achieving serum uric acid levels < 6.0 mg/dL and reducing serum uric acid levels in the period from baseline to final visit when compared to placebo and to allopurinol. However, the grade of evidence ranged from low to high, which indicates that further research is needed.
Conflict of interest statement
None known. This systematic review did not receive specific funding.
Figures































































































Update of
- doi: 10.1002/14651858.CD008653
Similar articles
-
Allopurinol for chronic gout.Cochrane Database Syst Rev. 2014 Oct 14;2014(10):CD006077. doi: 10.1002/14651858.CD006077.pub3. Cochrane Database Syst Rev. 2014. PMID: 25314636 Free PMC article. Review.
-
Interventions for tophi in gout.Cochrane Database Syst Rev. 2021 Aug 11;8(8):CD010069. doi: 10.1002/14651858.CD010069.pub3. Cochrane Database Syst Rev. 2021. PMID: 34379791 Free PMC article. Review.
-
A systematic review and meta-analysis on the safety and efficacy of febuxostat versus allopurinol in chronic gout.Semin Arthritis Rheum. 2013 Dec;43(3):367-75. doi: 10.1016/j.semarthrit.2013.05.004. Semin Arthritis Rheum. 2013. PMID: 24326033 Review.
-
Effectiveness and safety of different doses of febuxostat compared with allopurinol in the treatment of hyperuricemia: a meta-analysis of randomized controlled trials.BMC Pharmacol Toxicol. 2023 Dec 14;24(1):79. doi: 10.1186/s40360-023-00723-5. BMC Pharmacol Toxicol. 2023. PMID: 38098046 Free PMC article.
-
Uricosuric medications for chronic gout.Cochrane Database Syst Rev. 2014 Nov 14;2014(11):CD010457. doi: 10.1002/14651858.CD010457.pub2. Cochrane Database Syst Rev. 2014. PMID: 25392987 Free PMC article.
Cited by
-
Comparative efficacy and safety of urate-lowering therapy for the treatment of hyperuricemia: a systematic review and network meta-analysis.Sci Rep. 2016 Sep 8;6:33082. doi: 10.1038/srep33082. Sci Rep. 2016. PMID: 27605442 Free PMC article. Review.
-
Pharmacodynamic evaluation of the XOR inhibitor WN1703 in a model of chronic hyperuricemia in rats induced by yeast extract combined with potassium oxonate.Curr Res Pharmacol Drug Discov. 2022 Mar 27;3:100098. doi: 10.1016/j.crphar.2022.100098. eCollection 2022. Curr Res Pharmacol Drug Discov. 2022. PMID: 35465446 Free PMC article.
-
Safety and efficacy of oral febuxostat for treatment of HLA-B*5801-negative gout: a randomized, open-label, multicentre, allopurinol-controlled study.Scand J Rheumatol. 2016 Jul;45(4):304-11. doi: 10.3109/03009742.2015.1099729. Epub 2016 Jan 15. Scand J Rheumatol. 2016. PMID: 26771445 Free PMC article. Clinical Trial.
-
Serum uric acid levels and cardiovascular disease: the Gordian knot.J Thorac Dis. 2016 Nov;8(11):E1462-E1466. doi: 10.21037/jtd.2016.11.39. J Thorac Dis. 2016. PMID: 28066631 Free PMC article.
-
[Full version of the S2e guidelines on gouty arthritis : Evidence-based guidelines of the German Society of Rheumatology (DGRh)].Z Rheumatol. 2016 Aug;75 Suppl 2:11-60. doi: 10.1007/s00393-016-0147-6. Z Rheumatol. 2016. PMID: 27481119 Review. German. No abstract available.
References
References to studies included in this review
Becker 2005a {published data only}
-
- Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. New England Journal of Medicine 2005;353(23):2450‐61. [PUBMED: 16339094] - PubMed
Becker 2005b {published data only}
-
- Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, et alt L, Joseph‐Ridge N. Febuxostat, a Novel Nonpurine Selective Inhibitor ofXanthine Oxidase:a twenty‐eight‐day, multicenter, phase II, randomized, double‐blind, placebo‐controlled, dose‐response clinical trial examining safety and efficacy in patients with gout. Arthritis and Rheumatism 2005;52(3):916–23. [PUBMED: 15751090] - PubMed
-
- Goldfarb, D. S.MacDonald, P.Hunt, B.Gunawardhana, L. [Febuxostat in Gout: Serum Urate Responses in Uric Acid Overproducers vs. Underexcretors]. American Journal of Kidney Diseases. 2010; Vol. 55:B59. - PubMed
Becker 2009 {published data only}
-
- Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful long‐term urate lowering with febuxostat or allopurinol in subjects with gout. Journal of Rheumatology 2009;36(6):1273‐82. - PubMed
Becker 2010 {published data only}
-
- Whelton A, Becker MA, MacDonald P, Hunt B, Jackson RL. [Gout subjects with hyperuricemia and renal impairment treated withfebuxostat or allopurinol for 6 months]. International Journal of Rheumatic Diseases. 2010; Vol. 13:172‐7.
Schumacher 2008 {published data only}
-
- Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28‐week, phase III, randomized, double‐blind, parallel‐group trial. Arthritis and Rheumatism 2008;59(11):1540‐8. [PUBMED: 18975369] - PubMed
Schumacher 2009 {published data only}
-
- Schumacher HR, Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5‐yr findings of the FOCUS efficacy and safety study. Rheumatology 2009;48:188‐94. - PubMed
References to studies excluded from this review
Becker 2008 {published data only}
-
- Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph‐Ridge N. Determinants of the clinical outcomes of gout during the first year of urate‐lowering therapy. Nucleosides, Nucleotides & Nucleic Acids 2008;27(6):585‐91. - PubMed
Komoriya 2004 {published data only}
-
- Komoriya K, Hoshide S, Takeda K, Kobayashi H, Kubo J, Tsuchimoto M, et al. Pharmacokinetics and pharmacodynamics of febuxostat (TMX‐67), a non‐purine selective inhibitor of xanthine oxidase/xanthine dehydrogenase (NPSIXO) in patients with gout and/or hyperuricemia. Nucleosides, Nucleotides & Nucleic Acids 2004;23(8‐9):1119‐22. - PubMed
References to studies awaiting assessment
References to ongoing studies
NCT01078389 {unpublished data only}
-
- A Multicenter, Randomized, Double‐Blind, Phase 2 Study to Evaluate the Effect of Febuxostat Versus Placebo in Joint Damage in Hyperuricemic Subjects With Early Gout. Ongoing study March 2010.
NCT01082640 {unpublished data only}
-
- A Multicenter, Randomized, Double‐Blind, Phase 2 Study to Evaluate the Effect of Febuxostat Versus Placebo on Renal Function in Gout Subjects With Hyperuricemia and Moderate to Severe Renal Impairment. Ongoing study April 2010.
NCT01101035 {unpublished data only}
-
- A Multicenter, Randomized, Active‐Control, Phase 3B Study to Evaluate the Cardiovascular harms of Febuxostat and Allopurinol in Subjects With Gout and Cardiovascular Comorbidities. Ongoing study May 2010.
Additional references
Anderson 2010
Arellano 1993
-
- Arellano F, Sacristán JA. Allopurinol hypersensitivity syndrome: a review. Annals of Pharmacotherapy 1993;27(3):337‐43. [PUBMED: 8453174] - PubMed
Bhandari 2004
Bruce 2006
-
- Bruce SP. Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and gout. Annals of Pharmacotherapy 2006;40(12):2187‐94. [PUBMED: 17132810] - PubMed
Cates 2004
-
- Cates C. Visual Rx 2.0 NNT Calculator [Computer program]. Dr Chris Cates EBM Website www.nntonline.net 2004.
Chohan 2009
-
- Chohan S, Becker MA. Update on emerging urate‐lowering therapies. Current Opinion in Rheumatology 2009;21(2):143‐9. [PUBMED: 19339925] - PubMed
Dalbeth 2007
-
- Dalbeth N, Stamp L. Allopurinol dosing in renal impairment: Walking the tightrope between adequate urate lowering and adverse events. Seminars in Dialysis September–October 2007;20(5):391–5. - PubMed
Edwards 1981
-
- Edwards NL, Recker D, Airozo D, Fox IH. Enhanced purine salvage during allopurinol therapy: an important pharmacologic property in humans. Journal of Laboratory and Clinical Medicine 1981;98(5):673‐83. [PUBMED: 7299239] - PubMed
Edwards 2009
-
- Edwards NL. Febuxostat: a new treatment for hyperuricaemia in gout. Rheumatology (Oxford) 2009;48 Suppl 2:ii15‐9. [PUBMED: 19447778] - PubMed
EMEA 2008
-
- European Medicines Agency. European Public Assessment Report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_... April 21, 2012.
FDA 2009
-
- US Food, Drug Administration. Summary Review for Regulatory Action. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/021856s000_SumR.pdf February 13, 2009.
Higgins 2003
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jansen 2010
-
- Jansen TL, Richette P, Perez‐Ruiz F, Tausche AK, Guerne PA, Punzi L, et al. International position paper on febuxostat. Clinical Rheumatology 2010;29:835‐40. [PUBMED: 20401506] - PubMed
Jordan 2007
-
- Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group (SGAWG). British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford) 2007;46(8):1372‐4. [PUBMED: 17522099] - PubMed
Li‐Yu 2001
-
- Li‐Yu J, Clayburne G, Sieck M, Beutler A, Rull M, Eisner E, Schumacher HR Jr. Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout?. Journal of Rheumatology 2001;28(3):577‐80. [PUBMED: 11296962] - PubMed
Maxwell 2009
OMERACT 9
-
- Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. Journal of Rheumatology October 2008;36(10):2342‐5. - PubMed
Pascual 2007
Schlesinger 2004
-
- Schlesinger N. Management of acute and chronic gouty arthritis: present state‐of‐the‐art. Drugs 2004;64(21):2399‐416. [PUBMED: 15481999] - PubMed
Schumacher 2005
-
- Schumacher HR Jr, Edwards LN, Perez RF, Becker M, Chen LX, Furst DE, et al. Outcome measures for acute and chronic gout. Journal of Rheumatology 2005;32:2452–5. - PubMed
Singh 2010
Stevenson 2011
-
- Stevenson M, Pandor A. Febuxostat for the management of hyperuricaemia in patients with gout: a NICE single technology appraisal. Pharmacoeconomics 2011;29(2):133‐40. [PUBMED: 21155617] - PubMed
Wallace 1977
-
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis and Rheumatism 1977;20(3):895‐900. [PUBMED: 856219] - PubMed
Zhang 2006
-
- Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the Rheumatic Diseases 2006;65(10):1312‐24. [PUBMED: 16707532] - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

