Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen
- PMID: 23150545
- PMCID: PMC3511734
- DOI: 10.1073/pnas.1208927109
Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen
Abstract
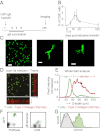
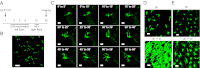
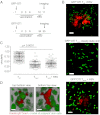
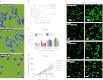
Recent work has demonstrated that following the clearance of infection a stable population of memory T cells remains present in peripheral organs and contributes to the control of secondary infections. However, little is known about how tissue-resident memory T cells behave in situ and how they encounter newly infected target cells. Here we demonstrate that antigen-specific CD8(+) T cells that remain in skin following herpes simplex virus infection show a steady-state crawling behavior in between keratinocytes. Spatially explicit simulations of the migration of these tissue-resident memory T cells indicate that the migratory dendritic behavior of these cells allows the detection of antigen-expressing target cells in physiologically relevant time frames of minutes to hours. Furthermore, we provide direct evidence for the identification of rare antigen-expressing epithelial cells by skin-patrolling memory T cells in vivo. These data demonstrate the existence of skin patrol by memory T cells and reveal the value of this patrol in the rapid detection of renewed infections at a previously infected site.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Local Antigen Encounter Is Essential for Establishing Persistent CD8+ T-Cell Memory in the CNS.Front Immunol. 2019 Mar 4;10:351. doi: 10.3389/fimmu.2019.00351. eCollection 2019. Front Immunol. 2019. PMID: 30886617 Free PMC article.
-
Tissue patrol by resident memory CD8+ T cells in human skin.Nat Immunol. 2019 Jun;20(6):756-764. doi: 10.1038/s41590-019-0404-3. Epub 2019 May 20. Nat Immunol. 2019. PMID: 31110315
-
Cross-presenting Langerhans cells are required for the early reactivation of resident CD8+ memory T cells in the epidermis.Proc Natl Acad Sci U S A. 2023 Aug 22;120(34):e2219932120. doi: 10.1073/pnas.2219932120. Epub 2023 Aug 14. Proc Natl Acad Sci U S A. 2023. PMID: 37579158 Free PMC article.
-
Armed and Ready: Transcriptional Regulation of Tissue-Resident Memory CD8 T Cells.Front Immunol. 2018 Jul 30;9:1770. doi: 10.3389/fimmu.2018.01770. eCollection 2018. Front Immunol. 2018. PMID: 30131803 Free PMC article. Review.
-
Tissue-resident lymphocytes: from adaptive to innate immunity.Cell Mol Immunol. 2019 Mar;16(3):205-215. doi: 10.1038/s41423-018-0192-y. Epub 2019 Jan 11. Cell Mol Immunol. 2019. PMID: 30635650 Free PMC article. Review.
Cited by
-
Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance.Nat Commun. 2015 May 19;6:7090. doi: 10.1038/ncomms8090. Nat Commun. 2015. PMID: 25987506 Free PMC article.
-
Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate.Science. 2019 Oct 11;366(6462):eaav5728. doi: 10.1126/science.aav5728. Science. 2019. PMID: 31601741 Free PMC article.
-
Runx3 drives a CD8+ T cell tissue residency program that is absent in CD4+ T cells.Nat Immunol. 2022 Aug;23(8):1236-1245. doi: 10.1038/s41590-022-01273-4. Epub 2022 Jul 26. Nat Immunol. 2022. PMID: 35882933
-
Niches for the Long-Term Maintenance of Tissue-Resident Memory T Cells.Front Immunol. 2018 May 31;9:1214. doi: 10.3389/fimmu.2018.01214. eCollection 2018. Front Immunol. 2018. PMID: 29904388 Free PMC article. Review.
-
Crawling and Gliding: A Computational Model for Shape-Driven Cell Migration.PLoS Comput Biol. 2015 Oct 21;11(10):e1004280. doi: 10.1371/journal.pcbi.1004280. eCollection 2015 Oct. PLoS Comput Biol. 2015. PMID: 26488304 Free PMC article.
References
-
- Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. - PubMed
-
- Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

