Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis
- PMID: 23147525
- PMCID: PMC3561464
- DOI: 10.1038/ajg.2012.363
Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis
Abstract
Objectives: Antibodies to infliximab (ATIs) have been associated with loss of clinical response and lower serum infliximab (IFX) levels in some studies of patients with inflammatory bowel disease (IBD). This has important implications for patient management and development of novel biologic therapies. The objective of this study was to perform a systematic review and meta-analysis of studies that reported clinical outcomes and IFX levels according to patients' ATI status.
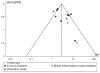
Methods: MEDLINE, Web of Science, CINAHL, Scopus, and EMBASE were searched for eligible studies. Quality assessment was undertaken using GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria. Raw data from studies meeting inclusion criteria was pooled for meta-analysis of effect estimates. Sensitivity analysis was performed for all outcomes. Funnel plot was performed to assess for publication bias.
Results: Thirteen studies met the inclusion criteria, and reported results in 1,378 patients with IBD. All included studies had a high risk of bias in at least one quality domain. The pooled risk ratio (RR) of loss of clinical response to IFX in patients with IBD who had ATIs was 3.2 (95% confidence interval (CI): 2.0-4.9, P<0.0001), when compared with patients without ATIs. This effect estimate was predominantly based on data from patients (N=494) with Crohn's disease (RR: 3.2, 95% CI: 1.9-5.5, P<0.0001). Data only from patients with ulcerative colitis (n=86) exhibited a non-significant RR of loss of response of 2.2 (95% CI: 0.5-9.0, P=0.3) in those with ATIs. Heterogeneity existed between studies, in both methods of ATI detection, and clinical outcomes reported. Three studies (n=243) reported trough serum IFX levels according to ATI status; the standardized mean difference in trough serum IFX levels between groups was -0.8 (95% CI -1.2, -0.4, P<0.0001). A funnel plot suggested the presence of publication bias.
Conclusions: The presence of ATIs is associated with a significantly higher risk of loss of clinical response to IFX and lower serum IFX levels in patients with IBD. Published studies on this topic lack uniform reporting of outcomes. High risk of bias was present in all the included studies.
Conflict of interest statement
Figures

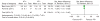
Similar articles
-
Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies.Inflamm Bowel Dis. 2012 Sep;18(9):1628-33. doi: 10.1002/ibd.21919. Epub 2011 Oct 29. Inflamm Bowel Dis. 2012. PMID: 22038899
-
A Real-life Population Pharmacokinetic Study Reveals Factors Associated with Clearance and Immunogenicity of Infliximab in Inflammatory Bowel Disease.Inflamm Bowel Dis. 2017 Apr;23(4):650-660. doi: 10.1097/MIB.0000000000001043. Inflamm Bowel Dis. 2017. PMID: 28195852
-
Utility of serum TNF-α, infliximab trough level, and antibody titers in inflammatory bowel disease.World J Gastroenterol. 2014 May 7;20(17):5031-5. doi: 10.3748/wjg.v20.i17.5031. World J Gastroenterol. 2014. PMID: 24833846 Free PMC article.
-
Systematic Review and Meta-Analysis: Serum Infliximab Levels During Maintenance Therapy and Outcomes in Inflammatory Bowel Disease.J Crohns Colitis. 2016 May;10(5):619-25. doi: 10.1093/ecco-jcc/jjw007. Epub 2016 Jan 13. J Crohns Colitis. 2016. PMID: 26763722 Free PMC article. Review.
-
Use of infliximab and anti-infliximab antibody measurements to evaluate and optimize efficacy and safety of infliximab maintenance therapy in Crohn's disease.Dan Med J. 2013 Apr;60(4):B4616. Dan Med J. 2013. PMID: 23651723 Review.
Cited by
-
Retrospective Database Analysis: Dose Escalation and Adherence in Patients Initiating Biologics for Ulcerative Colitis.Dig Dis. 2022;40(5):553-564. doi: 10.1159/000521299. Epub 2021 Dec 8. Dig Dis. 2022. PMID: 34879378 Free PMC article.
-
Efficacy of Combined Initial Treatment of Methotrexate with Infliximab in Pediatric Crohn's Disease: A Pilot Study.Biomedicines. 2023 Sep 19;11(9):2575. doi: 10.3390/biomedicines11092575. Biomedicines. 2023. PMID: 37761016 Free PMC article.
-
Crohn's disease.Nat Rev Dis Primers. 2020 Apr 2;6(1):22. doi: 10.1038/s41572-020-0156-2. Nat Rev Dis Primers. 2020. PMID: 32242028 Review.
-
Crohn's Disease: Evolution, Epigenetics, and the Emerging Role of Microbiome-Targeted Therapies.Curr Gastroenterol Rep. 2016 Mar;18(3):13. doi: 10.1007/s11894-016-0487-z. Curr Gastroenterol Rep. 2016. PMID: 26908281 Review.
-
The benefit of combination therapy depends on disease phenotype and duration in Crohn's disease.Aliment Pharmacol Ther. 2017 Jul;46(2):162-168. doi: 10.1111/apt.14125. Epub 2017 May 3. Aliment Pharmacol Ther. 2017. PMID: 28470787 Free PMC article.
References
-
- Talley NJ, Abreu MT, Achkar JP, et al. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106:S2–S25. - PubMed
-
- Clark M, Colombel JF, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology. 2007;133:312–39. - PubMed
-
- Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–98. - PubMed
-
- Danese S, Fiorino G, Reinisch W. Review article: causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-α therapy. Aliment Pharmacol Ther. 2011;34:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

