Constitutive β-catenin signaling by the viral chemokine receptor US28
- PMID: 23145028
- PMCID: PMC3493591
- DOI: 10.1371/journal.pone.0048935
Constitutive β-catenin signaling by the viral chemokine receptor US28
Abstract
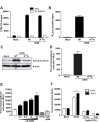
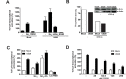
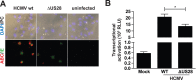
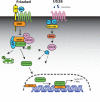
Chronic activation of Wnt/β-catenin signaling is found in a variety of human malignancies including melanoma, colorectal and hepatocellular carcinomas. Interestingly, expression of the HCMV-encoded chemokine receptor US28 in intestinal epithelial cells promotes intestinal neoplasia in transgenic mice, which is associated with increased nuclear accumulation of β-catenin. In this study we show that this viral receptor constitutively activates β-catenin and enhances β-catenin-dependent transcription. Our data illustrate that this viral receptor does not activate β-catenin via the classical Wnt/Frizzled signaling pathway. Analysis of US28 mediated signaling indicates the involvement of the Rho-Rho kinase (ROCK) pathway in the activation of β-catenin. Moreover, cells infected with HCMV show significant increases in β-catenin stabilization and signaling, which is mediated to a large extent by expression of US28. The modulation of the β-catenin signal transduction pathway by a viral chemokine receptor provides alternative regulation of this pathway, with potential relevance for the development of colon cancer and virus-associated diseases.
Conflict of interest statement
Figures

Similar articles
-
The human cytomegalovirus-encoded G protein-coupled receptor UL33 exhibits oncomodulatory properties.J Biol Chem. 2019 Nov 1;294(44):16297-16308. doi: 10.1074/jbc.RA119.007796. Epub 2019 Sep 13. J Biol Chem. 2019. PMID: 31519750 Free PMC article.
-
The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth.Oncogene. 2018 Jul;37(30):4110-4121. doi: 10.1038/s41388-018-0255-7. Epub 2018 Apr 30. Oncogene. 2018. PMID: 29706656 Free PMC article.
-
Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1α/PKM2 axis in glioblastoma cells.Oncotarget. 2016 Oct 18;7(42):67966-67985. doi: 10.18632/oncotarget.11817. Oncotarget. 2016. PMID: 27602585 Free PMC article.
-
Human Cytomegalovirus US28: a functionally selective chemokine binding receptor.Infect Disord Drug Targets. 2009 Nov;9(5):548-56. doi: 10.2174/187152609789105696. Infect Disord Drug Targets. 2009. PMID: 19594424 Free PMC article. Review.
-
US28: HCMV's Swiss Army Knife.Viruses. 2018 Aug 20;10(8):445. doi: 10.3390/v10080445. Viruses. 2018. PMID: 30127279 Free PMC article. Review.
Cited by
-
RhoB is a component of the human cytomegalovirus assembly complex and is required for efficient viral production.Cell Cycle. 2015;14(17):2748-63. doi: 10.1080/15384101.2015.1066535. Cell Cycle. 2015. PMID: 26114383 Free PMC article.
-
TRANSPIRE: A Computational Pipeline to Elucidate Intracellular Protein Movements from Spatial Proteomics Data Sets.J Am Soc Mass Spectrom. 2020 Jul 1;31(7):1422-1439. doi: 10.1021/jasms.0c00033. Epub 2020 May 29. J Am Soc Mass Spectrom. 2020. PMID: 32401031 Free PMC article.
-
Viruses in Cancers of the Digestive System: Active Contributors or Idle Bystanders?Int J Mol Sci. 2020 Oct 30;21(21):8133. doi: 10.3390/ijms21218133. Int J Mol Sci. 2020. PMID: 33143318 Free PMC article. Review.
-
The human cytomegalovirus-encoded G protein-coupled receptor UL33 exhibits oncomodulatory properties.J Biol Chem. 2019 Nov 1;294(44):16297-16308. doi: 10.1074/jbc.RA119.007796. Epub 2019 Sep 13. J Biol Chem. 2019. PMID: 31519750 Free PMC article.
-
Chemokine Subversion by Human Herpesviruses.J Innate Immun. 2018;10(5-6):465-478. doi: 10.1159/000492161. Epub 2018 Aug 30. J Innate Immun. 2018. PMID: 30165356 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

