Vaccines: from empirical development to rational design
- PMID: 23144616
- PMCID: PMC3493475
- DOI: 10.1371/journal.ppat.1003001
Vaccines: from empirical development to rational design
Abstract
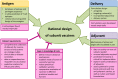
Infectious diseases are responsible for an overwhelming number of deaths worldwide and their clinical management is often hampered by the emergence of multi-drug-resistant strains. Therefore, prevention through vaccination currently represents the best course of action to combat them. However, immune escape and evasion by pathogens often render vaccine development difficult. Furthermore, most currently available vaccines were empirically designed. In this review, we discuss why rational design of vaccines is not only desirable but also necessary. We introduce recent developments towards specifically tailored antigens, adjuvants, and delivery systems, and discuss the methodological gaps and lack of knowledge still hampering true rational vaccine design. Finally, we address the potential and limitations of different strategies and technologies for advancing vaccine development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Principles of Vaccination.Methods Mol Biol. 2016;1403:57-84. doi: 10.1007/978-1-4939-3387-7_3. Methods Mol Biol. 2016. PMID: 27076125
-
Vaccine adjuvants: key tools for innovative vaccine design.Curr Top Med Chem. 2013;13(20):2562-80. doi: 10.2174/15680266113136660183. Curr Top Med Chem. 2013. PMID: 24066891 Review.
-
Novel Adjuvants and Immunomodulators for Veterinary Vaccines.Methods Mol Biol. 2016;1349:63-82. doi: 10.1007/978-1-4939-3008-1_5. Methods Mol Biol. 2016. PMID: 26458830
-
Molecular signatures of vaccine adjuvants.Vaccine. 2015 Sep 29;33(40):5302-7. doi: 10.1016/j.vaccine.2015.04.099. Epub 2015 May 16. Vaccine. 2015. PMID: 25989447 Review.
-
Vaccine adjuvants: smart components to boost the immune system.Arch Pharm Res. 2017 Nov;40(11):1238-1248. doi: 10.1007/s12272-017-0969-z. Epub 2017 Oct 13. Arch Pharm Res. 2017. PMID: 29027637 Review.
Cited by
-
Roles for Pathogen Interference in Influenza Vaccination, with Implications to Vaccine Effectiveness (VE) and Attribution of Influenza Deaths.Infect Dis Rep. 2022 Sep 23;14(5):710-758. doi: 10.3390/idr14050076. Infect Dis Rep. 2022. PMID: 36286197 Free PMC article. Review.
-
Myeloid-derived suppressor cells and vaccination against pathogens.Front Cell Infect Microbiol. 2022 Sep 29;12:1003781. doi: 10.3389/fcimb.2022.1003781. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36250061 Free PMC article. Review.
-
Microscale thermophoresis analysis of the molecular interaction between small nuclear ribonucleoprotein polypeptide G and the RING finger domain of RBBP6 towards anti-cancer drug discovery.Am J Transl Res. 2021 Nov 15;13(11):12775-12785. eCollection 2021. Am J Transl Res. 2021. PMID: 34956492 Free PMC article.
-
In Vivo Synthesis of Cyclic-di-GMP Using a Recombinant Adenovirus Preferentially Improves Adaptive Immune Responses against Extracellular Antigens.J Immunol. 2016 Feb 15;196(4):1741-52. doi: 10.4049/jimmunol.1501272. Epub 2016 Jan 20. J Immunol. 2016. PMID: 26792800 Free PMC article.
-
Vaccine skepticism and vaccine development stages; inoculation from "cowpox" lesion to the current mRNA vaccine of COVID-19: review.Ther Adv Vaccines Immunother. 2024 Oct 11;12:25151355241288135. doi: 10.1177/25151355241288135. eCollection 2024. Ther Adv Vaccines Immunother. 2024. PMID: 39399302 Free PMC article. Review.
References
-
- Bagnoli F, Baudner B, Mishra RP, Bartolini E, Fiaschi L, et al. (2011) Designing the next generation of vaccines for global public health. OMICS 15: 545–566. - PubMed
-
- Poland GA, Kennedy RB, Ovsyannikova IG (2011) Vaccinomics and personalized vaccinology: is science leading us toward a new path of directed vaccine development and discovery? PLoS Pathog 7: e1002344 doi:10.1371/journal.ppat.1002344 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

