A combination of local inflammation and central memory T cells potentiates immunotherapy in the skin
- PMID: 23144496
- PMCID: PMC3518562
- DOI: 10.4049/jimmunol.1200709
A combination of local inflammation and central memory T cells potentiates immunotherapy in the skin
Abstract
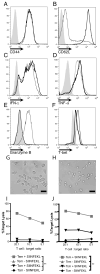
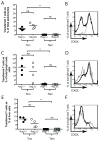
Adoptive T cell therapy uses the specificity of the adaptive immune system to target cancer and virally infected cells. Yet the mechanism and means by which to enhance T cell function are incompletely described, especially in the skin. In this study, we use a murine model of immunotherapy to optimize cell-mediated immunity in the skin. We show that in vitro-derived central but not effector memory-like T cells bring about rapid regression of skin-expressing cognate Ag as a transgene in keratinocytes. Local inflammation induced by the TLR7 receptor agonist imiquimod subtly yet reproducibly decreases time to skin graft rejection elicited by central but not effector memory T cells in an immunodeficient mouse model. Local CCL4, a chemokine liberated by TLR7 agonism, similarly enhances central memory T cell function. In this model, IL-2 facilitates the development in vivo of effector function from central memory but not effector memory T cells. In a model of T cell tolerogenesis, we further show that adoptively transferred central but not effector memory T cells can give rise to successful cutaneous immunity, which is dependent on a local inflammatory cue in the target tissue at the time of adoptive T cell transfer. Thus, adoptive T cell therapy efficacy can be enhanced if CD8(+) T cells with a central memory T cell phenotype are transferred, and IL-2 is present with contemporaneous local inflammation.
Figures

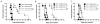



Similar articles
-
Lymphopenia-induced homeostatic proliferation of CD8+ T cells is a mechanism for effective allogeneic skin graft rejection following burn injury.J Immunol. 2006 Jun 1;176(11):6717-26. doi: 10.4049/jimmunol.176.11.6717. J Immunol. 2006. PMID: 16709831
-
Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets.J Immunol. 2004 Jan 15;172(2):857-63. doi: 10.4049/jimmunol.172.2.857. J Immunol. 2004. PMID: 14707056
-
Memory T cells originate from adoptively transferred effectors and reconstituting host cells after sequential lymphodepletion and adoptive immunotherapy.J Immunol. 2004 Mar 15;172(6):3462-8. doi: 10.4049/jimmunol.172.6.3462. J Immunol. 2004. PMID: 15004146
-
Immune modulation of inflammatory conditions: regulatory T cells for treatment of GvHD.Immunol Res. 2012 Sep;53(1-3):200-12. doi: 10.1007/s12026-012-8267-9. Immunol Res. 2012. PMID: 22418725 Review.
-
Pathophysiology of Skin Resident Memory T Cells.Front Immunol. 2021 Feb 3;11:618897. doi: 10.3389/fimmu.2020.618897. eCollection 2020. Front Immunol. 2021. PMID: 33633737 Free PMC article. Review.
Cited by
-
Effector and central memory T helper 2 cells respond differently to peptide immunotherapy.Proc Natl Acad Sci U S A. 2014 Feb 25;111(8):E784-93. doi: 10.1073/pnas.1316178111. Epub 2014 Feb 10. Proc Natl Acad Sci U S A. 2014. PMID: 24516158 Free PMC article.
-
Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice.Cancer Immunol Immunother. 2016 May;65(5):601-11. doi: 10.1007/s00262-016-1823-8. Epub 2016 Mar 24. Cancer Immunol Immunother. 2016. PMID: 27011014 Free PMC article.
-
HPV16-E7 expression in squamous epithelium creates a local immune suppressive environment via CCL2- and CCL5- mediated recruitment of mast cells.PLoS Pathog. 2014 Oct 23;10(10):e1004466. doi: 10.1371/journal.ppat.1004466. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25340820 Free PMC article.
-
Blockade of PD-1/PD-L1 promotes adoptive T-cell immunotherapy in a tolerogenic environment.PLoS One. 2015 Mar 5;10(3):e0119483. doi: 10.1371/journal.pone.0119483. eCollection 2015. PLoS One. 2015. PMID: 25741704 Free PMC article.
-
Impact of T cell selection methods in the success of clinical adoptive immunotherapy.Cell Mol Life Sci. 2014 Apr;71(7):1211-24. doi: 10.1007/s00018-013-1463-5. Cell Mol Life Sci. 2014. PMID: 24077876 Free PMC article.
References
-
- Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4:46–54. - PubMed
-
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. - PMC - PubMed
-
- Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, Shalmon B, Hardan I, Catane R, Markel G, Apter S, Ben-Nun A, Kuchuk I, Shimoni A, Nagler A, Schachter J. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

