The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening
- PMID: 23136376
- PMCID: PMC3531844
- DOI: 10.1105/tpc.112.103283
The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening
Abstract
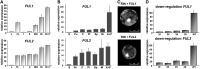
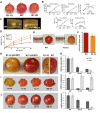
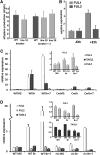
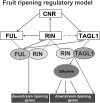
Tomato (Solanum lycopersicum) contains two close homologs of the Arabidopsis thaliana MADS domain transcription factor FRUITFULL (FUL), FUL1 (previously called TDR4) and FUL2 (previously MBP7). Both proteins interact with the ripening regulator RIPENING INHIBITOR (RIN) and are expressed during fruit ripening. To elucidate their function in tomato, we characterized single and double FUL1 and FUL2 knockdown lines. Whereas the single lines only showed very mild alterations in fruit pigmentation, the double silenced lines exhibited an orange-ripe fruit phenotype due to highly reduced lycopene levels, suggesting that FUL1 and FUL2 have a redundant function in fruit ripening. More detailed analyses of the phenotype, transcriptome, and metabolome of the fruits silenced for both FUL1 and FUL2 suggest that the genes are involved in cell wall modification, the production of cuticle components and volatiles, and glutamic acid (Glu) accumulation. Glu is responsible for the characteristic umami taste of the present-day cultivated tomato fruit. In contrast with previously identified ripening regulators, FUL1 and FUL2 do not regulate ethylene biosynthesis but influence ripening in an ethylene-independent manner. Our data combined with those of others suggest that FUL1/2 and TOMATO AGAMOUS-LIKE1 regulate different subsets of the known RIN targets, probably in a protein complex with the latter.
Figures
Similar articles
-
Transcriptional regulation of fruit ripening by tomato FRUITFULL homologs and associated MADS box proteins.Plant Cell. 2014 Jan;26(1):89-101. doi: 10.1105/tpc.113.119453. Epub 2014 Jan 10. Plant Cell. 2014. PMID: 24415769 Free PMC article.
-
Tomato FRUITFULL homologues act in fruit ripening via forming MADS-box transcription factor complexes with RIN.Plant Mol Biol. 2013 Jul;82(4-5):427-38. doi: 10.1007/s11103-013-0071-y. Epub 2013 May 16. Plant Mol Biol. 2013. PMID: 23677393
-
Tomato FRUITFULL homologs regulate fruit ripening via ethylene biosynthesis.Biosci Biotechnol Biochem. 2014;78(2):231-7. doi: 10.1080/09168451.2014.878221. Epub 2014 Apr 14. Biosci Biotechnol Biochem. 2014. PMID: 25036675
-
Variations on a theme in fruit development: the PLE lineage of MADS-box genes in tomato (TAGL1) and other species.Planta. 2017 Aug;246(2):313-321. doi: 10.1007/s00425-017-2725-5. Epub 2017 Jun 28. Planta. 2017. PMID: 28660293 Review.
-
Transcriptional control of fleshy fruit development and ripening.J Exp Bot. 2014 Aug;65(16):4527-41. doi: 10.1093/jxb/eru316. J Exp Bot. 2014. PMID: 25080453 Review.
Cited by
-
Transcriptional Activity of the MADS Box ARLEQUIN/TOMATO AGAMOUS-LIKE1 Gene Is Required for Cuticle Development of Tomato Fruit.Plant Physiol. 2015 Jul;168(3):1036-48. doi: 10.1104/pp.15.00469. Epub 2015 May 27. Plant Physiol. 2015. PMID: 26019301 Free PMC article.
-
Ethylene response factor ERF.D7 activates auxin response factor 2 paralogs to regulate tomato fruit ripening.Plant Physiol. 2022 Nov 28;190(4):2775-2796. doi: 10.1093/plphys/kiac441. Plant Physiol. 2022. PMID: 36130295 Free PMC article.
-
Seed shattering: from models to crops.Front Plant Sci. 2015 Jun 24;6:476. doi: 10.3389/fpls.2015.00476. eCollection 2015. Front Plant Sci. 2015. PMID: 26157453 Free PMC article. Review.
-
Activating glutamate decarboxylase activity by removing the autoinhibitory domain leads to hyper γ-aminobutyric acid (GABA) accumulation in tomato fruit.Plant Cell Rep. 2017 Jan;36(1):103-116. doi: 10.1007/s00299-016-2061-4. Epub 2016 Oct 4. Plant Cell Rep. 2017. PMID: 27704232
-
Transcriptional Regulation of Carotenoid Biosynthesis in Plants: So Many Regulators, So Little Consensus.Front Plant Sci. 2019 Aug 9;10:1017. doi: 10.3389/fpls.2019.01017. eCollection 2019. Front Plant Sci. 2019. PMID: 31447877 Free PMC article. Review.
References
-
- Aker J., Borst J.W., Karlova R., de Vries S. (2006). The Arabidopsis thaliana AAA protein CDC48A interacts in vivo with the somatic embryogenesis receptor-like kinase 1 receptor at the plasma membrane. J. Struct. Biol. 156: 62–71 - PubMed
-
- Barry C.S., Blume B., Bouzayen M., Cooper W., Hamilton A.J., Grierson D. (1996). Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 9: 525–535 - PubMed
-
- Barry C.S., Giovannoni J.J. (2007). Ethylene and fruit ripening. J. Plant Growth Regul. 26: 143–159
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

