Inhibition of Porphyromonas gingivalis-induced periodontal bone loss by CXCR4 antagonist treatment
- PMID: 23134610
- PMCID: PMC3494490
- DOI: 10.1111/j.2041-1014.2012.00657.x
Inhibition of Porphyromonas gingivalis-induced periodontal bone loss by CXCR4 antagonist treatment
Abstract
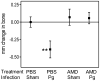
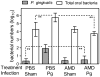
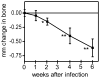
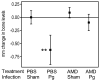
Microbial pathogens have evolved mechanisms to proactively manipulate innate immunity, thereby improving their fitness in mammalian hosts. We have previously shown that Porphyromonas gingivalis exploits CXC-chemokine receptor-4 (CXCR4) to instigate a subversive crosstalk with Toll-like receptor 2 that inhibits leukocyte killing of this periodontal pathogen. However, whether CXCR4 plays a role in periodontal disease pathogenesis has not been previously addressed. Here, we hypothesized that CXCR4 is required for P. gingivalis virulence in the periodontium and that treatment with AMD3100, a potent CXCR4 antagonist, would inhibit P. gingivalis-induced periodontitis. Indeed, mice given AMD3100 via osmotic minipumps became resistant to induction of periodontal bone loss following oral inoculation with P. gingivalis. AMD3100 appeared to act in an antimicrobial manner, because mice treated with AMD3100 were protected against P. gingivalis colonization and the associated elevation of the total microbiota counts in the periodontal tissue. Moreover, even when administered 2 weeks after infection, AMD3100 halted the progression of P. gingivalis-induced periodontal bone loss. Therefore, AMD3100 can act in both preventive and therapeutic ways and CXCR4 antagonism could be a promising novel approach to treat human periodontitis.
© 2012 John Wiley & Sons A/S.
Figures
Similar articles
-
Mechanism and implications of CXCR4-mediated integrin activation by Porphyromonas gingivalis.Mol Oral Microbiol. 2013 Aug;28(4):239-49. doi: 10.1111/omi.12021. Epub 2013 Jan 21. Mol Oral Microbiol. 2013. PMID: 23331495 Free PMC article.
-
CXCR4 signaling contributes to alveolar bone resorption in Porphyromonas gingivalis-induced periodontitis in mice.J Oral Sci. 2017 Dec 27;59(4):571-577. doi: 10.2334/josnusd.16-0830. Epub 2017 Oct 31. J Oral Sci. 2017. PMID: 29093284
-
CXCR4 signaling in macrophages contributes to periodontal mechanical hypersensitivity in Porphyromonas gingivalis-induced periodontitis in mice.Mol Pain. 2017 Jan;13:1744806916689269. doi: 10.1177/1744806916689269. Mol Pain. 2017. PMID: 28326928 Free PMC article.
-
Porphyromonas gingivalis mediated periodontal disease and atherosclerosis: disparate diseases with commonalities in pathogenesis through TLRs.Curr Pharm Des. 2007;13(36):3665-75. doi: 10.2174/138161207783018554. Curr Pharm Des. 2007. PMID: 18220804 Review.
-
Pathogenesis of Important Virulence Factors of Porphyromonas gingivalis via Toll-Like Receptors.Front Cell Infect Microbiol. 2019 Jul 18;9:262. doi: 10.3389/fcimb.2019.00262. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31380305 Free PMC article. Review.
Cited by
-
Porphyromonas gingivalis outside the oral cavity.Odontology. 2022 Jan;110(1):1-19. doi: 10.1007/s10266-021-00647-8. Epub 2021 Aug 19. Odontology. 2022. PMID: 34410562 Review.
-
Brucella targets the host ubiquitin-specific protease, Usp8, through the effector protein, TcpB, for facilitating infection of macrophages.Infect Immun. 2024 Feb 13;92(2):e0028923. doi: 10.1128/iai.00289-23. Epub 2024 Jan 4. Infect Immun. 2024. PMID: 38174929 Free PMC article.
-
Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis.Cell Host Microbe. 2014 Jun 11;15(6):768-78. doi: 10.1016/j.chom.2014.05.012. Cell Host Microbe. 2014. PMID: 24922578 Free PMC article.
-
Salivary microbiomes: a potent evidence in forensic investigations.Forensic Sci Med Pathol. 2024 Sep;20(3):1058-1065. doi: 10.1007/s12024-023-00759-3. Epub 2024 Jan 4. Forensic Sci Med Pathol. 2024. PMID: 38175312 Review.
-
Mechanism and implications of CXCR4-mediated integrin activation by Porphyromonas gingivalis.Mol Oral Microbiol. 2013 Aug;28(4):239-49. doi: 10.1111/omi.12021. Epub 2013 Jan 21. Mol Oral Microbiol. 2013. PMID: 23331495 Free PMC article.
References
-
- Assuma R, Oates T, Cochran D, et al. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. - PubMed
-
- Chaves ES, Jeffcoat MK, Ryerson CC, Snyder B. Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J Clin Periodontol. 2000;27:897–903. - PubMed
-
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. - PubMed
-
- De Clercq E. Potential clinical applications of the CXCR4 antagonist bicyclam AMD3100. Mini Rev Med Chem. 2005;5:805–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

