p21-Activated kinase 6 (PAK6) inhibits prostate cancer growth via phosphorylation of androgen receptor and tumorigenic E3 ligase murine double minute-2 (Mdm2)
- PMID: 23132866
- PMCID: PMC3561555
- DOI: 10.1074/jbc.M112.384289
p21-Activated kinase 6 (PAK6) inhibits prostate cancer growth via phosphorylation of androgen receptor and tumorigenic E3 ligase murine double minute-2 (Mdm2)
Abstract
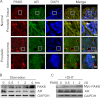
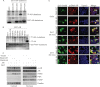
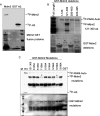
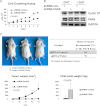
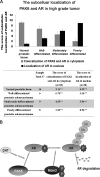
The androgen receptor (AR) signaling pathway plays a crucial role in the development and growth of prostate malignancies. Regulation of AR homeostasis in prostate tumorigenesis has not yet been fully characterized. In this study, we demonstrate that p21-activated kinase 6 (PAK6) inhibits prostate tumorigenesis by regulating AR homeostasis. First, we demonstrated that in normal prostate epithelium, AR co-localizes with PAK6 in the cytoplasm and translocates into the nucleus in malignant prostate. Furthermore, AR phosphorylation at Ser-578 by PAK6 promotes AR-E3 ligase murine double minute-2 (Mdm2) association, causing AR degradation upon androgen stimuli. We also showed that PAK6 phosphorylates Mdm2 on Thr-158 and Ser-186, which is critical for AR ubiquitin-mediated degradation. Moreover, we found that Thr-158 collaborates with Ser-186 for AR-Mdm2 association and AR ubiquitin-mediated degradation as it facilitates PAK6-mediated AR homeostasis. PAK6 knockdown promotes prostate tumor growth in vivo. Interestingly, we found a strong inverse correlation between PAK6 and AR expression in the cytoplasm of prostate cancer cells. These observations indicate that PAK6 may be important for the maintenance of androgen-induced AR signaling homeostasis and in prostate malignancy, as well as being a possible new therapeutic target for AR-positive and hormone-sensitive prostate cancer.
Figures



Comment in
-
Re: p21-activated kinase 6 (PAK6) inhibits prostate cancer growth via phosphorylation of androgen receptor and tumorigenic E3 ligase murine double minute-2 (Mdm2).J Urol. 2013 Sep;190(3):1131. doi: 10.1016/j.juro.2013.05.069. Epub 2013 Jun 5. J Urol. 2013. PMID: 23931236 No abstract available.
Similar articles
-
Re: p21-activated kinase 6 (PAK6) inhibits prostate cancer growth via phosphorylation of androgen receptor and tumorigenic E3 ligase murine double minute-2 (Mdm2).J Urol. 2013 Sep;190(3):1131. doi: 10.1016/j.juro.2013.05.069. Epub 2013 Jun 5. J Urol. 2013. PMID: 23931236 No abstract available.
-
Differential regulation of androgen receptor by PIM-1 kinases via phosphorylation-dependent recruitment of distinct ubiquitin E3 ligases.J Biol Chem. 2012 Jun 29;287(27):22959-68. doi: 10.1074/jbc.M111.338350. Epub 2012 May 14. J Biol Chem. 2012. PMID: 22584579 Free PMC article.
-
Direct interaction between AR and PAK6 in androgen-stimulated PAK6 activation.PLoS One. 2013 Oct 10;8(10):e77367. doi: 10.1371/journal.pone.0077367. eCollection 2013. PLoS One. 2013. PMID: 24130878 Free PMC article.
-
Mechanism of p21-activated kinase 6-mediated inhibition of androgen receptor signaling.J Biol Chem. 2004 Jan 16;279(3):1922-31. doi: 10.1074/jbc.M311145200. Epub 2003 Oct 22. J Biol Chem. 2004. PMID: 14573606
-
Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation.Cell Cycle. 2007 Mar 15;6(6):647-51. doi: 10.4161/cc.6.6.4028. Epub 2007 Mar 21. Cell Cycle. 2007. PMID: 17387277 Review.
Cited by
-
INPP4B suppresses prostate cancer cell invasion.Cell Commun Signal. 2014 Sep 25;12:61. doi: 10.1186/s12964-014-0061-y. Cell Commun Signal. 2014. PMID: 25248616 Free PMC article.
-
Co-activator candidate interactions for orphan nuclear receptor NR2E1.BMC Genomics. 2016 Oct 26;17(1):832. doi: 10.1186/s12864-016-3173-5. BMC Genomics. 2016. PMID: 27782803 Free PMC article.
-
p-21 Activated Kinase as a Molecular Target for Chemoprevention in Diabetes.Geriatrics (Basel). 2018 Oct 19;3(4):73. doi: 10.3390/geriatrics3040073. Geriatrics (Basel). 2018. PMID: 31011108 Free PMC article. Review.
-
Ethanol extract of Vanilla planifolia stems reduces PAK6 expression and induces cell death in glioblastoma cells.J Cell Mol Med. 2024 Sep;28(17):e70065. doi: 10.1111/jcmm.70065. J Cell Mol Med. 2024. PMID: 39233332 Free PMC article.
-
Proline-Directed Androgen Receptor Phosphorylation.J Mol Genet Med. 2013 Oct;7(3):75. doi: 10.4172/1747-0862.1000075. J Mol Genet Med. 2013. PMID: 25866551 Free PMC article.
References
-
- Agoulnik I. U., Weigel N. L. (2008) Androgen receptor coactivators and prostate cancer. Adv. Exp. Med. Biol. 617, 245–255 - PubMed
-
- Buchanan G., Irvine R. A., Coetzee G. A., Tilley W. D. (2001) Contribution of the androgen receptor to prostate cancer predisposition and progression. Cancer Metastasis Rev. 20, 207–223 - PubMed
-
- Hobisch A., Culig Z., Radmayr C., Bartsch G., Klocker H., Hittmair A. (1996) Androgen receptor status of lymph node metastases from prostate cancer. Prostate 28, 129–135 - PubMed
-
- Ruizeveld de Winter J. A., Janssen P. J., Sleddens H. M., Verleun-Mooijman M. C., Trapman J., Brinkmann A. O., Santerse A. B., Schröder F. H., van der Kwast T. H. (1994) Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am. J. Pathol. 144, 735–746 - PMC - PubMed
-
- Saraon P., Jarvi K., Diamandis E. P. (2011) Molecular alterations during progression of prostate cancer to androgen independence. Clin. Chem. 57, 1366–1375 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

