Cysteine 70 of ankyrin-G is S-palmitoylated and is required for function of ankyrin-G in membrane domain assembly
- PMID: 23129772
- PMCID: PMC3527982
- DOI: 10.1074/jbc.M112.417501
Cysteine 70 of ankyrin-G is S-palmitoylated and is required for function of ankyrin-G in membrane domain assembly
Abstract
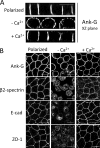
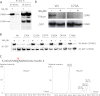
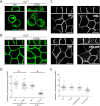
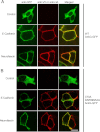
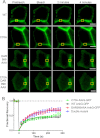
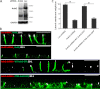
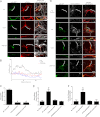
Ankyrin-G (AnkG) coordinates protein composition of diverse membrane domains, including epithelial lateral membranes and neuronal axon initial segments. However, how AnkG itself localizes to these membrane domains is not understood. We report that AnkG remains on the plasma membrane in Madin-Darby canine kidney (MDCK) cells grown in low calcium, although these cells lack apical-basal polarity and exhibit loss of plasma membrane association of AnkG partners, E-cadherin and β(2)-spectrin. We subsequently demonstrate using mutagenesis and mass spectrometry that AnkG is S-palmitoylated exclusively at Cys-70, which is located in a loop of the first ankyrin repeat and is conserved in the vertebrate ankyrin family. Moreover, C70A mutation abolishes membrane association of 190-kDa AnkG in MDCK cells grown in low calcium. C70A 190-kDa AnkG fails to restore biogenesis of epithelial lateral membranes in MDCK cells depleted of endogenous AnkG. In addition, C70A 270-kDa AnkG fails to cluster at the axon initial segment of AnkG-depleted cultured hippocampal neurons and fails to recruit neurofascin as well as voltage-gated sodium channels. These effects of C70A mutation combined with evidence for its S-palmitoylation are consistent with a requirement of palmitoylation for targeting and function of AnkG in membrane domain biogenesis at epithelial lateral membranes and neuronal axon initial segments.
Figures

Similar articles
-
Structural basis for the membrane association of ankyrinG via palmitoylation.Sci Rep. 2016 Apr 5;6:23981. doi: 10.1038/srep23981. Sci Rep. 2016. PMID: 27046665 Free PMC article.
-
The largest isoform of Ankyrin-G is required for lattice structure of the axon initial segment.Biochem Biophys Res Commun. 2021 Nov 12;578:28-34. doi: 10.1016/j.bbrc.2021.09.017. Epub 2021 Sep 8. Biochem Biophys Res Commun. 2021. PMID: 34534742
-
An Adaptable Spectrin/Ankyrin-Based Mechanism for Long-Range Organization of Plasma Membranes in Vertebrate Tissues.Curr Top Membr. 2016;77:143-84. doi: 10.1016/bs.ctm.2015.10.001. Epub 2015 Nov 30. Curr Top Membr. 2016. PMID: 26781832 Review.
-
Giant ankyrin-G: a critical innovation in vertebrate evolution of fast and integrated neuronal signaling.Proc Natl Acad Sci U S A. 2015 Jan 27;112(4):957-64. doi: 10.1073/pnas.1416544112. Epub 2014 Dec 31. Proc Natl Acad Sci U S A. 2015. PMID: 25552556 Free PMC article.
-
Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels.J Neurocytol. 1999 Apr-May;28(4-5):303-18. doi: 10.1023/a:1007005528505. J Neurocytol. 1999. PMID: 10739573 Review.
Cited by
-
Physical and functional convergence of the autism risk genes Scn2a and Ank2 in neocortical pyramidal cell dendrites.Neuron. 2024 Apr 3;112(7):1133-1149.e6. doi: 10.1016/j.neuron.2024.01.003. Epub 2024 Jan 29. Neuron. 2024. PMID: 38290518
-
Is There a Link Between the Pathogenic Human Coronavirus Envelope Protein and Immunopathology? A Review of the Literature.Front Microbiol. 2020 Sep 3;11:2086. doi: 10.3389/fmicb.2020.02086. eCollection 2020. Front Microbiol. 2020. PMID: 33013759 Free PMC article. Review.
-
Dual ankyrinG and subpial autoantibodies in a man with well-controlled HIV infection with steroid-responsive meningoencephalitis: A case report.Front Neurol. 2023 Jan 23;13:1102484. doi: 10.3389/fneur.2022.1102484. eCollection 2022. Front Neurol. 2023. PMID: 36756346 Free PMC article.
-
Roles of palmitoylation in structural long-term synaptic plasticity.Mol Brain. 2021 Jan 11;14(1):8. doi: 10.1186/s13041-020-00717-y. Mol Brain. 2021. PMID: 33430908 Free PMC article. Review.
-
CDK5/p35-Dependent Microtubule Reorganization Contributes to Homeostatic Shortening of the Axon Initial Segment.J Neurosci. 2023 Jan 18;43(3):359-372. doi: 10.1523/JNEUROSCI.0917-22.2022. Epub 2022 Dec 6. J Neurosci. 2023. PMID: 36639893 Free PMC article.
References
-
- Bennett V., Healy J. (2008) Organizing the fluid membrane bilayer. Diseases linked to spectrin and ankyrin. Trends Mol. Med. 14, 28–36 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

