Down-regulation of 8-oxoguanine DNA glycosylase 1 expression in the airway epithelium ameliorates allergic lung inflammation
- PMID: 23127499
- PMCID: PMC3678389
- DOI: 10.1016/j.dnarep.2012.10.002
Down-regulation of 8-oxoguanine DNA glycosylase 1 expression in the airway epithelium ameliorates allergic lung inflammation
Abstract
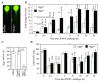
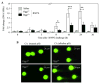
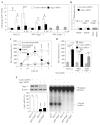
Allergic airway inflammation is characterized by increased expression of pro-inflammatory mediators, inflammatory cell infiltration, mucus hypersecretion, and airway hyperresponsiveness, in parallel with oxidative DNA base and strand damage, whose etiological role is not understood. Our goal was to establish the role of 8-oxoguanine (8-oxoG), a common oxidatively damaged base, and its repair by 8-oxoguanine DNA glycosylase 1 (Ogg1) in allergic airway inflammatory processes. Airway inflammation was induced by intranasally administered ragweed (Ambrosia artemisiifolia) pollen grain extract (RWPE) in sensitized BALB/c mice. We utilized siRNA technology to deplete Ogg1 from airway epithelium; 8-oxoG and DNA strand break levels were quantified by Comet assays. Inflammatory cell infiltration and epithelial methaplasia were determined histologically, mucus and cytokines levels biochemically and enhanced pause was used as the main index of airway hyperresponsiveness. Decreased Ogg1 expression and thereby 8-oxoG repair in the airway epithelium conveyed a lower inflammatory response after RWPE challenge of sensitized mice, as determined by expression of Th2 cytokines, eosinophilia, epithelial methaplasia, and airway hyperresponsiveness. In contrast, 8-oxoG repair in Ogg1-proficient airway epithelium was coupled to an increase in DNA single-strand break (SSB) levels and exacerbation of allergen challenge-dependent inflammation. Decreased expression of the Nei-like glycosylases Neil1 and Neil2 that preferentially excise ring-opened purines and 5-hydroxyuracil, respectively, did not alter the above parameters of allergic immune responses to RWPE. These results show that DNA SSBs formed during Ogg1-mediated repair of 8-oxoG augment antigen-driven allergic immune responses. A transient modulation of OGG1 expression/activity in airway epithelial cells could have clinical benefits.
Copyright © 2012 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Innate inflammation induced by the 8-oxoguanine DNA glycosylase-1-KRAS-NF-κB pathway.J Immunol. 2014 Nov 1;193(9):4643-53. doi: 10.4049/jimmunol.1401625. Epub 2014 Sep 29. J Immunol. 2014. PMID: 25267977 Free PMC article.
-
Oxidized base 8-oxoguanine, a product of DNA repair processes, contributes to dendritic cell activation.Free Radic Biol Med. 2019 Nov 1;143:209-220. doi: 10.1016/j.freeradbiomed.2019.08.010. Epub 2019 Aug 10. Free Radic Biol Med. 2019. PMID: 31408726 Free PMC article.
-
Pollen-induced oxidative DNA damage response regulates miRNAs controlling allergic inflammation.Am J Physiol Lung Cell Mol Physiol. 2017 Dec 1;313(6):L1058-L1068. doi: 10.1152/ajplung.00141.2017. Epub 2017 Aug 10. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28798252 Free PMC article.
-
Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases.Free Radic Biol Med. 2017 Jun;107:179-201. doi: 10.1016/j.freeradbiomed.2016.11.042. Epub 2016 Nov 27. Free Radic Biol Med. 2017. PMID: 27903453 Review.
-
Lost in the Crowd: How Does Human 8-Oxoguanine DNA Glycosylase 1 (OGG1) Find 8-Oxoguanine in the Genome?Int J Mol Sci. 2020 Nov 7;21(21):8360. doi: 10.3390/ijms21218360. Int J Mol Sci. 2020. PMID: 33171795 Free PMC article. Review.
Cited by
-
The potential for OGG1 inhibition to be a therapeutic strategy for pulmonary diseases.Expert Opin Ther Targets. 2024 Mar;28(3):117-130. doi: 10.1080/14728222.2024.2317900. Epub 2024 Feb 14. Expert Opin Ther Targets. 2024. PMID: 38344773 Review.
-
Small-molecule-mediated OGG1 inhibition attenuates pulmonary inflammation and lung fibrosis in a murine lung fibrosis model.Nat Commun. 2023 Feb 6;14(1):643. doi: 10.1038/s41467-023-36314-5. Nat Commun. 2023. PMID: 36746968 Free PMC article.
-
Small-molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation.Science. 2018 Nov 16;362(6416):834-839. doi: 10.1126/science.aar8048. Science. 2018. PMID: 30442810 Free PMC article.
-
Small Molecule Inhibitors of 8-Oxoguanine DNA Glycosylase-1 (OGG1).ACS Chem Biol. 2015 Oct 16;10(10):2334-43. doi: 10.1021/acschembio.5b00452. Epub 2015 Aug 7. ACS Chem Biol. 2015. PMID: 26218629 Free PMC article.
-
Small-Molecule Inhibitor of 8-Oxoguanine DNA Glycosylase 1 Regulates Inflammatory Responses during Pseudomonas aeruginosa Infection.J Immunol. 2020 Oct 15;205(8):2231-2242. doi: 10.4049/jimmunol.1901533. Epub 2020 Sep 14. J Immunol. 2020. PMID: 32929043 Free PMC article.
References
-
- Vrtala S, Grote M, Duchene M, van Ree R, Kraft D, Scheiner O, Valenta R. Properties of tree and grass pollen allergens: reinvestigation of the linkage between solubility and allergenicity. Int Arch Allergy Immunol. 1993;102:160–169. - PubMed
-
- D’Amato G, Liccardi G, D’Amato M, Cazzola M. Outdoor air pollution, climatic changes and allergic bronchial asthma. Eur Respir J. 2002;20:763–776. - PubMed
-
- Annesi-Maesano I, Rouve S, Desqueyroux H, Jankovski R, Klossek JM, Thibaudon M, Demoly P, Didier A. Grass Pollen Counts, Air Pollution Levels and Allergic Rhinitis Severity. Int Arch Allergy Immunol. 158:397–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

