Primordial germ cells in mice
- PMID: 23125014
- PMCID: PMC3536339
- DOI: 10.1101/cshperspect.a008375
Primordial germ cells in mice
Abstract
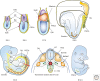
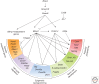
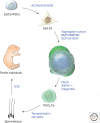
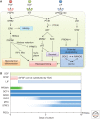
Germ cell development creates totipotency through genetic as well as epigenetic regulation of the genome function. Primordial germ cells (PGCs) are the first germ cell population established during development and are immediate precursors for both the oocytes and spermatogonia. We here summarize recent findings regarding the mechanism of PGC development in mice. We focus on the transcriptional and signaling mechanism for PGC specification, potential pluripotency, and epigenetic reprogramming in PGCs and strategies for the reconstitution of germ cell development using pluripotent stem cells in culture. Continued studies on germ cell development may lead to the generation of totipotency in vitro, which should have a profound influence on biological science as well as on medicine.
Figures

Similar articles
-
In or out stemness: comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells.Curr Stem Cell Res Ther. 2009 May;4(2):87-97. doi: 10.2174/157488809788167391. Curr Stem Cell Res Ther. 2009. PMID: 19442193 Review.
-
Specification of germ cell fate in mice.Philos Trans R Soc Lond B Biol Sci. 2003 Aug 29;358(1436):1363-70. doi: 10.1098/rstb.2003.1324. Philos Trans R Soc Lond B Biol Sci. 2003. PMID: 14511483 Free PMC article.
-
Human primordial germ cell commitment in vitro associates with a unique PRDM14 expression profile.EMBO J. 2015 Apr 15;34(8):1009-24. doi: 10.15252/embj.201488049. Epub 2015 Mar 6. EMBO J. 2015. PMID: 25750208 Free PMC article.
-
Germ cell specification in mice: signaling, transcription regulation, and epigenetic consequences.Reproduction. 2010 Jun;139(6):931-42. doi: 10.1530/REP-10-0043. Epub 2010 Apr 6. Reproduction. 2010. PMID: 20371640 Review.
-
Primordial germ cell specification: a context-dependent cellular differentiation event [corrected].Philos Trans R Soc Lond B Biol Sci. 2014 Dec 5;369(1657):20130543. doi: 10.1098/rstb.2013.0543. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25349452 Free PMC article. Review.
Cited by
-
Germline stem cells in human.Signal Transduct Target Ther. 2022 Oct 2;7(1):345. doi: 10.1038/s41392-022-01197-3. Signal Transduct Target Ther. 2022. PMID: 36184610 Free PMC article. Review.
-
Induction of mouse germ-cell fate by transcription factors in vitro.Nature. 2013 Sep 12;501(7466):222-6. doi: 10.1038/nature12417. Epub 2013 Aug 4. Nature. 2013. PMID: 23913270
-
Postnatal oogenesis leads to an exceptionally large ovarian reserve in naked mole-rats.Nat Commun. 2023 Feb 21;14(1):670. doi: 10.1038/s41467-023-36284-8. Nat Commun. 2023. PMID: 36810851 Free PMC article.
-
Comments on 'In vitro culture of cynomolgus monkey embryos beyond early gastrulation'.J Mol Cell Biol. 2020 Jun 11;12(5):400-402. doi: 10.1093/jmcb/mjz108. J Mol Cell Biol. 2020. PMID: 31863115 Free PMC article. No abstract available.
-
Blastula stage specification of avian neural crest.Dev Biol. 2020 Feb 1;458(1):64-74. doi: 10.1016/j.ydbio.2019.10.007. Epub 2019 Oct 11. Dev Biol. 2020. PMID: 31610145 Free PMC article.
References
-
- Abe K, Naruse C, Kato T, Nishiuchi T, Saitou M, Asano M 2011. Loss of heterochromatin protein 1γ reduces the number of primordial germ cells via impaired cell cycle progression in mice. Biol Reprod 85: 1013–1024 - PubMed
-
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T, Surani MA 2006. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol 8: 623–630 - PubMed
-
- Anderson R, Fassler R, Georges-Labouesse E, Hynes RO, Bader BL, Kreidberg JA, Schaible K, Heasman J, Wylie C 1999. Mouse primordial germ cells lacking β1 integrins enter the germline but fail to migrate normally to the gonads. Development 126: 1655–1664 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
