Structural asymmetry in the magnesium channel CorA points to sequential allosteric regulation
- PMID: 23112165
- PMCID: PMC3503211
- DOI: 10.1073/pnas.1209018109
Structural asymmetry in the magnesium channel CorA points to sequential allosteric regulation
Abstract
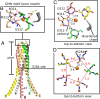
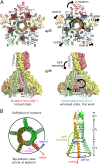
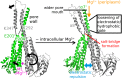
Magnesium ions (Mg(2+)) are essential for life, but the mechanisms regulating their transport into and out of cells remain poorly understood. The CorA-Mrs2-Alr1 superfamily of Mg(2+) channels represents the most prevalent group of proteins enabling Mg(2+) ions to cross membranes. Thermotoga maritima CorA (TmCorA) is the only member of this protein family whose complete 3D fold is known. Here, we report the crystal structure of a mutant in the presence and absence of divalent ions and compare it with previous divalent ion-bound TmCorA structures. With Mg(2+) present, this structure shows binding of a hydrated Mg(2+) ion to the periplasmic Gly-Met-Asn (GMN) motif, revealing clues of ion selectivity in this unique channel family. In the absence of Mg(2+), TmCorA displays an unexpected asymmetric conformation caused by radial and lateral tilts of protomers that leads to bending of the central, pore-lining helix. Molecular dynamics simulations support these movements, including a bell-like deflection. Mass spectrometric analysis confirms that major proteolytic cleavage occurs within a region that is selectively exposed by such a bell-like bending motion. Our results point to a sequential allosteric model of regulation, where intracellular Mg(2+) binding locks TmCorA in a symmetric, transport-incompetent conformation and loss of intracellular Mg(2+) causes an asymmetric, potentially influx-competent conformation of the channel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Exploring the structure and function of Thermotoga maritima CorA reveals the mechanism of gating and ion selectivity in Co2+/Mg2+ transport.Biochem J. 2013 May 1;451(3):365-74. doi: 10.1042/BJ20121745. Biochem J. 2013. PMID: 23425532 Free PMC article.
-
Probing structure-function relationships and gating mechanisms in the CorA Mg2+ transport system.J Biol Chem. 2008 Apr 25;283(17):11721-33. doi: 10.1074/jbc.M707889200. Epub 2008 Feb 14. J Biol Chem. 2008. PMID: 18276588
-
Real time dynamics of Gating-Related conformational changes in CorA.Elife. 2019 Nov 27;8:e47322. doi: 10.7554/eLife.47322. Elife. 2019. PMID: 31774394 Free PMC article.
-
The structure and regulation of magnesium selective ion channels.Biochim Biophys Acta. 2013 Nov;1828(11):2778-92. doi: 10.1016/j.bbamem.2013.08.002. Epub 2013 Aug 15. Biochim Biophys Acta. 2013. PMID: 23954807 Review.
-
The CorA family: structure and function revisited.Cell Mol Life Sci. 2007 Oct;64(19-20):2564-74. doi: 10.1007/s00018-007-7174-z. Cell Mol Life Sci. 2007. PMID: 17619822 Free PMC article. Review.
Cited by
-
Hyphal Growth in Trichosporon asahii Is Accelerated by the Addition of Magnesium.Microbiol Spectr. 2023 Jun 15;11(3):e0424222. doi: 10.1128/spectrum.04242-22. Epub 2023 Apr 27. Microbiol Spectr. 2023. PMID: 37102973 Free PMC article.
-
MRS2 missense variation at Asp216 abrogates inhibitory Mg2+ binding, potentiating cell migration and apoptosis resistance.Protein Sci. 2024 Aug;33(8):e5108. doi: 10.1002/pro.5108. Protein Sci. 2024. PMID: 38989547 Free PMC article.
-
Exploring the structure and function of Thermotoga maritima CorA reveals the mechanism of gating and ion selectivity in Co2+/Mg2+ transport.Biochem J. 2013 May 1;451(3):365-74. doi: 10.1042/BJ20121745. Biochem J. 2013. PMID: 23425532 Free PMC article.
-
The Cryo-EM structure of the CorA channel from Methanocaldococcus jannaschii in low magnesium conditions.Biochim Biophys Acta. 2015 Oct;1848(10 Pt A):2206-15. doi: 10.1016/j.bbamem.2015.06.002. Epub 2015 Jun 4. Biochim Biophys Acta. 2015. PMID: 26051127 Free PMC article.
-
Molecular basis of Mg2+ permeation through the human mitochondrial Mrs2 channel.Nat Commun. 2023 Aug 5;14(1):4713. doi: 10.1038/s41467-023-40516-2. Nat Commun. 2023. PMID: 37543649 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

