Iron regulator hepcidin exhibits antiviral activity against hepatitis C virus
- PMID: 23110054
- PMCID: PMC3478283
- DOI: 10.1371/journal.pone.0046631
Iron regulator hepcidin exhibits antiviral activity against hepatitis C virus
Abstract
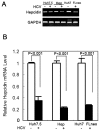
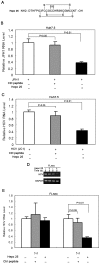
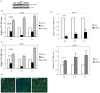
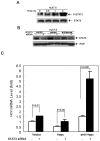
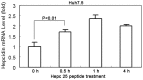
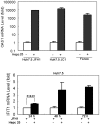
Hepatitis C viral infection affects 170 million people worldwide. It causes serious chronic liver diseases. HCV infection has been implicated in iron accumulation in the liver and iron overload has been shown to be a potential cofactor for HCV associated hepatocellular carcinoma progression. The underlying mechanisms are not understood. Human hepcidin, a 25 amino acid peptide mainly produced by hepatocytes, is a key regulator of iron metabolism. Alteration of hepcidin expression levels has been reported in the setting of chronic HCV infection and hepatocellular carcinoma. In this study, we aim to examine the interactions between HCV infection and hepcidin expression in liver cells. We found that hepcidin expression was suppressed in HCV infected cells. The suppressive effect appears to be regulated by histone acetylation but not DNA methylation. Moreover, we found that hepcidin had a direct antiviral activity against HCV replication in cell culture. The antiviral effect is associated with STAT3 activation. In conclusion, hepcidin can induce intracellular antiviral state while HCV has a strategy to suppress hepcidin expression. This may be a novel mechanism by which HCV circumvents hepatic innate antiviral defense.
Conflict of interest statement
Figures

Similar articles
-
Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity.Hepatology. 2008 Nov;48(5):1420-9. doi: 10.1002/hep.22486. Hepatology. 2008. PMID: 18671304
-
Hepcidin levels and iron status in beta-thalassemia major patients with hepatitis C virus infection.Egypt J Immunol. 2010;17(2):33-44. Egypt J Immunol. 2010. PMID: 23082485
-
Low hepcidin triggers hepatic iron accumulation in patients with hepatitis C.Nephrol Dial Transplant. 2014 Jun;29(6):1141-4. doi: 10.1093/ndt/gft467. Epub 2013 Nov 27. Nephrol Dial Transplant. 2014. PMID: 24286977
-
[How Histone Deacetylase 3 Controls Hepcidin Expression and Hepatitis C Virus Replication].Mol Biol (Mosk). 2023 May-Jun;57(3):427-439. Mol Biol (Mosk). 2023. PMID: 37326046 Review. Russian.
-
Relationship between Hepatitis C Virus Infection and Iron Overload.Chin Med J (Engl). 2017 Apr 5;130(7):866-871. doi: 10.4103/0366-6999.202737. Chin Med J (Engl). 2017. PMID: 28345552 Free PMC article. Review.
Cited by
-
The Role of Iron and Iron Overload in Chronic Liver Disease.Med Sci Monit. 2016 Jun 22;22:2144-51. doi: 10.12659/msm.896494. Med Sci Monit. 2016. PMID: 27332079 Free PMC article. Review.
-
Antimicrobial peptides from fish.Pharmaceuticals (Basel). 2014 Mar 3;7(3):265-310. doi: 10.3390/ph7030265. Pharmaceuticals (Basel). 2014. PMID: 24594555 Free PMC article.
-
[Progress on epigenetic regulation of iron homeostasis].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 May 25;49(1):58-70. doi: 10.3785/j.issn.1008-9292.2020.02.25. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 32621410 Free PMC article. Review. Chinese.
-
Hepatitis C virus infection causes iron deficiency in Huh7.5.1 cells.PLoS One. 2013 Dec 13;8(12):e83307. doi: 10.1371/journal.pone.0083307. eCollection 2013. PLoS One. 2013. PMID: 24349485 Free PMC article.
-
Serum iron markers in patients with chronic hepatitis C infection.Hepat Mon. 2013 Oct 5;13(10):e13136. doi: 10.5812/hepatmon.13136. eCollection 2013. Hepat Mon. 2013. PMID: 24348638 Free PMC article.
References
-
- Lauer GM, Walker BD (2001) Hepatitis C virus infection. N Engl J Med 345: 41–52. - PubMed
-
- Shepard CW, Finelli L, Alter MJ (2005) Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 5: 558–567. - PubMed
-
- Charlton M (2001) Hepatitis C infection in liver transplantation. Am J Transplant 1: 197–203. - PubMed
-
- Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, et al. (2000) LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 480: 147–150. - PubMed
-
- Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

