Metalloproteinases and their associated genes contribute to the functional integrity and noise-induced damage in the cochlear sensory epithelium
- PMID: 23100416
- PMCID: PMC3521496
- DOI: 10.1523/JNEUROSCI.1588-12.2012
Metalloproteinases and their associated genes contribute to the functional integrity and noise-induced damage in the cochlear sensory epithelium
Abstract
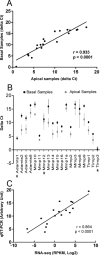
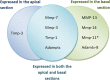
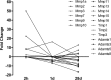
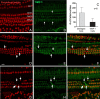
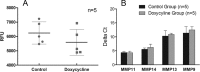
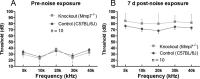
Matrix metalloproteinases (MMPs) and their related gene products regulate essential cellular functions. An imbalance in MMPs has been implicated in various neurological disorders, including traumatic injuries. Here, we report a role for MMPs and their related gene products in the modulation of cochlear responses to acoustic trauma in rats. The normal cochlea was shown to be enriched in MMP enzymatic activity, and this activity was reduced in a time-dependent manner after traumatic noise injury. The analysis of gene expression by RNA sequencing and qRT-PCR revealed the differential expression of MMPs and their related genes between functionally specialized regions of the sensory epithelium. The expression of these genes was dynamically regulated between the acute and chronic phases of noise-induced hearing loss. Moreover, noise-induced expression changes in two endogenous MMP inhibitors, Timp1 and Timp2, in sensory cells were dependent on the stage of nuclear condensation, suggesting a specific role for MMP activity in sensory cell apoptosis. A short-term application of doxycycline, a broad-spectrum inhibitor of MMPs, before noise exposure reduced noise-induced hearing loss and sensory cell death. In contrast, a 7 d treatment compromised hearing sensitivity and potentiated noise-induced hearing loss. This detrimental effect of the long-term inhibition of MMPs on noise-induced hearing loss was further confirmed using targeted Mmp7 knock-out mice. Together, these observations suggest that MMPs and their related genes participate in the regulation of cochlear responses to acoustic overstimulation and that the modulation of MMP activity can serve as a novel therapeutic target for the reduction of noise-induced cochlear damage.
Figures


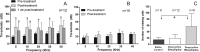


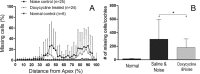
Similar articles
-
Matrix metalloproteinase-2 and -9 contribute to functional integrity and noise‑induced damage to the blood-labyrinth-barrier.Mol Med Rep. 2017 Aug;16(2):1731-1738. doi: 10.3892/mmr.2017.6784. Epub 2017 Jun 15. Mol Med Rep. 2017. PMID: 28627704 Free PMC article.
-
Noise-Induced Loss of Hair Cells and Cochlear Synaptopathy Are Mediated by the Activation of AMPK.J Neurosci. 2016 Jul 13;36(28):7497-510. doi: 10.1523/JNEUROSCI.0782-16.2016. J Neurosci. 2016. PMID: 27413159 Free PMC article.
-
[Noise-induced blood-labyrinth-barrier trauma of guinea pig and the protective effect of matrix metalloproteinase inhibitors].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020 Apr 7;55(4):363-370. doi: 10.3760/cma.j.cn115330-20190424-00303. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020. PMID: 32306634 Chinese.
-
Murine cochlear damage models in the context of hair cell regeneration research.Hear Res. 2024 Jun;447:109021. doi: 10.1016/j.heares.2024.109021. Epub 2024 Apr 27. Hear Res. 2024. PMID: 38703432 Review.
-
Genetics of noise-induced hearing loss in the mouse model.Hear Res. 2022 Nov;425:108505. doi: 10.1016/j.heares.2022.108505. Epub 2022 Apr 9. Hear Res. 2022. PMID: 35525820 Review.
Cited by
-
Molecular mechanisms and roles of inflammatory responses on low-frequency residual hearing after cochlear implantation.J Otol. 2022 Jan;17(1):54-58. doi: 10.1016/j.joto.2021.03.001. Epub 2021 Mar 10. J Otol. 2022. PMID: 35140760 Free PMC article. Review.
-
Prospective cohort study reveals MMP-9, a neuroplasticity regulator, as a prediction marker of cochlear implantation outcome in prelingual deafness treatment.Mol Neurobiol. 2022 Apr;59(4):2190-2203. doi: 10.1007/s12035-022-02732-7. Epub 2022 Jan 21. Mol Neurobiol. 2022. PMID: 35061219 Free PMC article.
-
Molecular profile of cochlear immunity in the resident cells of the organ of Corti.J Neuroinflammation. 2014 Oct 14;11:173. doi: 10.1186/s12974-014-0173-8. J Neuroinflammation. 2014. PMID: 25311735 Free PMC article.
-
Involvement of NLRP3-inflammasome pathway in noise-induced hearing loss.Neural Regen Res. 2022 Dec;17(12):2750-2754. doi: 10.4103/1673-5374.339499. Neural Regen Res. 2022. PMID: 35662224 Free PMC article.
-
Toll-like receptor 4 modulates the cochlear immune response to acoustic injury.Cell Death Dis. 2016 Jun 2;7(6):e2245. doi: 10.1038/cddis.2016.156. Cell Death Dis. 2016. PMID: 27253409 Free PMC article.
References
-
- Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38:308–317. - PubMed
-
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. - PubMed
-
- Bohne BA, Yohman L, Gruner MM. Cochlear damage following interrupted exposure to high-frequency noise. Hear Res. 1987;29:251–264. - PubMed
-
- Bohne BA, Harding GW, Lee SC. Death pathways in noise-damaged outer hair cells. Hear Res. 2007;223:61–70. - PubMed
-
- Burggraf D, Trinkl A, Dichgans M, Hamann GF. Doxycycline inhibits MMPs via modulation of plasminogen activators in focal cerebral ischemia. Neurobiol Dis. 2007;25:506–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
