IL-1R signalling is critical for regulation of multi-walled carbon nanotubes-induced acute lung inflammation in C57Bl/6 mice
- PMID: 23094697
- PMCID: PMC4080682
- DOI: 10.3109/17435390.2012.744110
IL-1R signalling is critical for regulation of multi-walled carbon nanotubes-induced acute lung inflammation in C57Bl/6 mice
Abstract
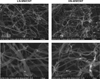
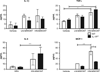
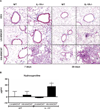
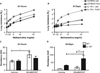
Exposure to certain engineered nanomaterials has been associated with pathological changes in animal models raising concerns about potential human health effects. MWCNT have been reported to activate the NLRP3 inflammasome in vitro, correlating with lung inflammation and pathology, in vivo. In this study, we investigated the role of IL-1 signalling in pulmonary inflammatory responses in WT and IL-1R-/- mice after exposure to MWCNT. The results suggest that MWCNT were effective in inducing acute pulmonary inflammation. Additionally, WT mice demonstrated significant increased airway resistance 24 h post exposure to MWCNT, which was also blocked in the IL-1R-/- mice. In contrast, by 28 days post exposure to MWCNT, the inflammatory response that was initially absent in IL-1R-/- mice was elevated in comparison to the WT mice. These data suggest that IL-1R signalling plays a crucial role in the regulation of MWCNT-induced pulmonary inflammation.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures

Similar articles
-
IL-33 modulates chronic airway resistance changes induced by multi-walled carbon nanotubes.Inhal Toxicol. 2014 Mar;26(4):240-9. doi: 10.3109/08958378.2014.880202. Epub 2014 Feb 6. Inhal Toxicol. 2014. PMID: 24502429 Free PMC article.
-
Mouse pulmonary dose- and time course-responses induced by exposure to nitrogen-doped multi-walled carbon nanotubes.Inhal Toxicol. 2020 Jan;32(1):24-38. doi: 10.1080/08958378.2020.1723746. Epub 2020 Feb 7. Inhal Toxicol. 2020. PMID: 32028803 Free PMC article.
-
Stat-6 signaling pathway and not Interleukin-1 mediates multi-walled carbon nanotube-induced lung fibrosis in mice: insights from an adverse outcome pathway framework.Part Fibre Toxicol. 2017 Sep 13;14(1):37. doi: 10.1186/s12989-017-0218-0. Part Fibre Toxicol. 2017. PMID: 28903780 Free PMC article.
-
Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes.Toxicology. 2010 Mar 10;269(2-3):136-47. doi: 10.1016/j.tox.2009.10.017. Epub 2009 Oct 24. Toxicology. 2010. PMID: 19857541
-
A Single Aspiration of Rod-like Carbon Nanotubes Induces Asbestos-like Pulmonary Inflammation Mediated in Part by the IL-1 Receptor.Toxicol Sci. 2015 Sep;147(1):140-55. doi: 10.1093/toxsci/kfv112. Epub 2015 Jun 5. Toxicol Sci. 2015. PMID: 26048651
Cited by
-
A novel human 3D lung microtissue model for nanoparticle-induced cell-matrix alterations.Part Fibre Toxicol. 2019 Apr 3;16(1):15. doi: 10.1186/s12989-019-0298-0. Part Fibre Toxicol. 2019. PMID: 30943996 Free PMC article.
-
Time-dependent subcellular distribution and effects of carbon nanotubes in lungs of mice.PLoS One. 2015 Jan 23;10(1):e0116481. doi: 10.1371/journal.pone.0116481. eCollection 2015. PLoS One. 2015. PMID: 25615613 Free PMC article.
-
IL-33 modulates chronic airway resistance changes induced by multi-walled carbon nanotubes.Inhal Toxicol. 2014 Mar;26(4):240-9. doi: 10.3109/08958378.2014.880202. Epub 2014 Feb 6. Inhal Toxicol. 2014. PMID: 24502429 Free PMC article.
-
STAT1-dependent and -independent pulmonary allergic and fibrogenic responses in mice after exposure to tangled versus rod-like multi-walled carbon nanotubes.Part Fibre Toxicol. 2017 Jul 17;14(1):26. doi: 10.1186/s12989-017-0207-3. Part Fibre Toxicol. 2017. PMID: 28716119 Free PMC article.
-
MyD88 mediates in vivo effector functions of alveolar macrophages in acute lung inflammatory responses to carbon nanotube exposure.Toxicol Appl Pharmacol. 2015 Nov 1;288(3):322-9. doi: 10.1016/j.taap.2015.08.004. Epub 2015 Aug 10. Toxicol Appl Pharmacol. 2015. PMID: 26272622 Free PMC article.
References
-
- Chen CH, Su HC, Chuang SC, Yen SJ, Chen YC, Lee YT, et al. Hydrophilic modification of neural microelectrode arrays based on multi-walled carbon nanotubes. Nanotechnology. 2010;21:485501. - PubMed
-
- Churg A, Zhou S, Wang X, Wang R, Wright JL. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol. 2009;40:482–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
