Neural crest stem cells from dental tissues: a new hope for dental and neural regeneration
- PMID: 23093977
- PMCID: PMC3472918
- DOI: 10.1155/2012/103503
Neural crest stem cells from dental tissues: a new hope for dental and neural regeneration
Abstract
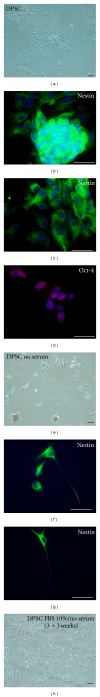
Several stem cell sources persist in the adult human body, which opens the doors to both allogeneic and autologous cell therapies. Tooth tissues have proven to be a surprisingly rich and accessible source of neural crest-derived ectomesenchymal stem cells (EMSCs), which may be employed to repair disease-affected oral tissues in advanced regenerative dentistry. Additionally, one area of medicine that demands intensive research on new sources of stem cells is nervous system regeneration, since this constitutes a therapeutic hope for patients affected by highly invalidating conditions such as spinal cord injury, stroke, or neurodegenerative diseases. However, endogenous adult sources of neural stem cells present major drawbacks, such as their scarcity and complicated obtention. In this context, EMSCs from dental tissues emerge as good alternative candidates, since they are preserved in adult human individuals, and retain both high proliferation ability and a neural-like phenotype in vitro. In this paper, we discuss some important aspects of tissue regeneration by cell therapy and point out some advantages that EMSCs provide for dental and neural regeneration. We will finally review some of the latest research featuring experimental approaches and benefits of dental stem cell therapy.
Figures
Similar articles
-
Applicability of tooth derived stem cells in neural regeneration.Neural Regen Res. 2016 Nov;11(11):1704-1707. doi: 10.4103/1673-5374.194705. Neural Regen Res. 2016. PMID: 28123398 Free PMC article. Review.
-
Comparative Craniofacial Bone Regeneration Capacities of Mesenchymal Stem Cells Derived from Human Neural Crest Stem Cells and Bone Marrow.ACS Biomater Sci Eng. 2021 Jan 11;7(1):207-221. doi: 10.1021/acsbiomaterials.0c00878. Epub 2020 Dec 9. ACS Biomater Sci Eng. 2021. PMID: 33455206
-
Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration.Neural Regen Res. 2020 Mar;15(3):373-381. doi: 10.4103/1673-5374.266043. Neural Regen Res. 2020. PMID: 31571644 Free PMC article. Review.
-
Neural crest-derived dental stem cells--where we are and where we are going.J Dent. 2014 Sep;42(9):1043-51. doi: 10.1016/j.jdent.2014.04.007. Epub 2014 Apr 24. J Dent. 2014. PMID: 24769107 Review.
-
Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration.Braz Dent J. 2011;22(2):91-8. doi: 10.1590/s0103-64402011000200001. Braz Dent J. 2011. PMID: 21537580 Review.
Cited by
-
Dental stem cells and their promising role in neural regeneration: an update.Clin Oral Investig. 2013 Dec;17(9):1969-83. doi: 10.1007/s00784-013-1030-3. Epub 2013 Jul 12. Clin Oral Investig. 2013. PMID: 23846214 Review.
-
SOX10-MITF pathway activity in melanoma cells.Arch Med Sci. 2017 Oct;13(6):1493-1503. doi: 10.5114/aoms.2016.60655. Epub 2016 Jun 17. Arch Med Sci. 2017. PMID: 29181082 Free PMC article.
-
Is There Such a Thing as a Genuine Cancer Stem Cell Marker? Perspectives from the Gut, the Brain and the Dental Pulp.Biology (Basel). 2020 Nov 27;9(12):426. doi: 10.3390/biology9120426. Biology (Basel). 2020. PMID: 33260962 Free PMC article. Review.
-
Characterization of Neurogenic Potential of Dental Pulp Stem Cells Cultured in Xeno/Serum-Free Condition: In Vitro and In Vivo Assessment.Stem Cells Int. 2016;2016:6921097. doi: 10.1155/2016/6921097. Epub 2016 Sep 5. Stem Cells Int. 2016. PMID: 27688776 Free PMC article.
-
Investigate the Odontogenic Differentiation and Dentin-Pulp Tissue Regeneration Potential of Neural Crest Cells.Front Bioeng Biotechnol. 2020 Jun 5;8:475. doi: 10.3389/fbioe.2020.00475. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32582651 Free PMC article.
References
-
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. The New England Journal of Medicine. 2010;363(2):147–155. - PubMed
-
- Hayani A, Lampeter E, Viswanatha D, Morgan D, Salvi SN. First report of autologous cord blood transplantation in the treatment of a child with leukemia. Pediatrics. 2007;119(1):e296–e300. - PubMed
-
- Greenow K, Clarke AR. Controlling the stem cell compartment and regeneration in vivo: the role of pluripotency pathways. Physiological Reviews. 2012;92(1):75–99. - PubMed
-
- Teri L. Behavior and caregiver burden: behavioral problems in patients with Alzheimer disease and its association with caregiver distress. Alzheimer Disease and Associated Disorders. 1997;11(4):S35–S38. - PubMed
-
- Lopez OL. The growing burden of Alzheimer's disease. The American Journal of Managed Care. 2011;17(supplement 13):S339–S345. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

