Intercellular calcium signaling in a gap junction-coupled cell network establishes asymmetric neuronal fates in C. elegans
- PMID: 23093425
- PMCID: PMC3478688
- DOI: 10.1242/dev.083428
Intercellular calcium signaling in a gap junction-coupled cell network establishes asymmetric neuronal fates in C. elegans
Abstract
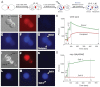
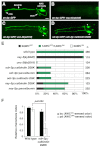
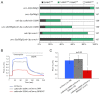
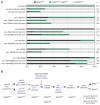
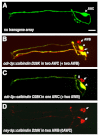
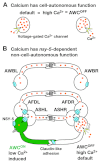
The C. elegans left and right AWC olfactory neurons specify asymmetric subtypes, one default AWC(OFF) and one induced AWC(ON), through a stochastic, coordinated cell signaling event. Intercellular communication between AWCs and non-AWC neurons via a NSY-5 gap junction network coordinates AWC asymmetry. However, the nature of intercellular signaling across the network and how individual non-AWC cells in the network influence AWC asymmetry is not known. Here, we demonstrate that intercellular calcium signaling through the NSY-5 gap junction neural network coordinates a precise 1AWC(ON)/1AWC(OFF) decision. We show that NSY-5 gap junctions in C. elegans cells mediate small molecule passage. We expressed vertebrate calcium-buffer proteins in groups of cells in the network to reduce intracellular calcium levels, thereby disrupting intercellular communication. We find that calcium in non-AWC cells of the network promotes the AWC(ON) fate, in contrast to the autonomous role of calcium in AWCs to promote the AWC(OFF) fate. In addition, calcium in specific non-AWCs promotes AWC(ON) side biases through NSY-5 gap junctions. Our results suggest a novel model in which calcium has dual roles within the NSY-5 network: autonomously promoting AWC(OFF) and non-autonomously promoting AWC(ON).
Figures
Similar articles
-
An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans.Cell. 2007 May 18;129(4):787-99. doi: 10.1016/j.cell.2007.02.052. Cell. 2007. PMID: 17512411
-
The microRNA mir-71 inhibits calcium signaling by targeting the TIR-1/Sarm1 adaptor protein to control stochastic L/R neuronal asymmetry in C. elegans.PLoS Genet. 2012;8(8):e1002864. doi: 10.1371/journal.pgen.1002864. Epub 2012 Aug 2. PLoS Genet. 2012. PMID: 22876200 Free PMC article.
-
SLO BK Potassium Channels Couple Gap Junctions to Inhibition of Calcium Signaling in Olfactory Neuron Diversification.PLoS Genet. 2016 Jan 15;12(1):e1005654. doi: 10.1371/journal.pgen.1005654. eCollection 2016 Jan. PLoS Genet. 2016. PMID: 26771544 Free PMC article.
-
Stochastic left-right neuronal asymmetry in Caenorhabditis elegans.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150407. doi: 10.1098/rstb.2015.0407. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821536 Free PMC article. Review.
-
Asymmetric neural development in the Caenorhabditis elegans olfactory system.Genesis. 2014 Jun;52(6):544-54. doi: 10.1002/dvg.22744. Epub 2014 Feb 7. Genesis. 2014. PMID: 24478264 Free PMC article. Review.
Cited by
-
Left-right asymmetry: lessons from Cancún.Development. 2013 Nov;140(22):4465-70. doi: 10.1242/dev.097907. Development. 2013. PMID: 24194469 Free PMC article.
-
NLR-1/CASPR Anchors F-Actin to Promote Gap Junction Formation.Dev Cell. 2020 Dec 7;55(5):574-587.e3. doi: 10.1016/j.devcel.2020.10.020. Epub 2020 Nov 24. Dev Cell. 2020. PMID: 33238150 Free PMC article.
-
The relationship between intraflagellar transport and upstream protein trafficking pathways and macrocyclic lactone resistance in Caenorhabditis elegans.G3 (Bethesda). 2024 Mar 6;14(3):jkae009. doi: 10.1093/g3journal/jkae009. G3 (Bethesda). 2024. PMID: 38227795 Free PMC article.
-
Multiple Subthreshold GPCR Signals Combined by the G-Proteins Gαq and Gαs Activate the Caenorhabditis elegans Egg-Laying Muscles.J Neurosci. 2023 May 24;43(21):3789-3806. doi: 10.1523/JNEUROSCI.2301-22.2023. Epub 2023 Apr 13. J Neurosci. 2023. PMID: 37055179 Free PMC article.
-
iPSC-Derived Trabecular Meshwork Cells Stimulate Endogenous TM Cell Division Through Gap Junction in a Mouse Model of Glaucoma.Invest Ophthalmol Vis Sci. 2021 Aug 2;62(10):28. doi: 10.1167/iovs.62.10.28. Invest Ophthalmol Vis Sci. 2021. PMID: 34427623 Free PMC article.
References
-
- Bargmann C. I., Hartwieg E., Horvitz H. R. (1993). Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515-527 - PubMed
-
- Bauer Huang S. L., Saheki Y., VanHoven M. K., Torayama I., Ishihara T., Katsura I., van der Linden A., Sengupta P., Bargmann C. I. (2007). Left-right olfactory asymmetry results from antagonistic functions of voltage-activated calcium channels and the Raw repeat protein OLRN-1 in C. elegans. Neural Dev. 2, 24 - PMC - PubMed
-
- Bennett M. V., Zukin R. S. (2004). Electrical coupling and neuronal synchronization in the mammalian brain. Neuron 41, 495-511 - PubMed
-
- Blomstrand F., Khatibi S., Muyderman H., Hansson E., Olsson T., Rönnbäck L. (1999). 5-Hydroxytryptamine and glutamate modulate velocity and extent of intercellular calcium signalling in hippocampal astroglial cells in primary cultures. Neuroscience 88, 1241-1253 - PubMed
-
- Boitano S., Dirksen E. R., Sanderson M. J. (1992). Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science 258, 292-295 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

