Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis
- PMID: 23092115
- PMCID: PMC3585742
- DOI: 10.1089/scd.2012.0495
Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis
Abstract
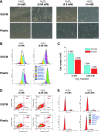
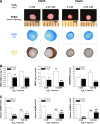
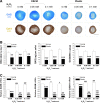
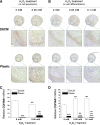
Clinical treatment of cartilage defects is challenging due to concomitant post-traumatic joint inflammation. This study was to demonstrate that the antioxidant ability of human adult synovium-derived stem cells (SDSCs) could be enhanced by ex vivo expansion on a decellularized stem cell matrix (DSCM). Microarray was used to evaluate oxidative, antioxidative, and chondrogenic status in SDSCs after expansion on the DSCM and induction in the chondrogenic medium. Hydrogen peroxide (H2O2) was added to create oxidative stress in either expanded SDSCs or chondrogenically induced premature pellets. The effect of H2O2 on SDSC proliferation was evaluated using flow cytometry. Chondrogenic differentiation of expanded SDSCs was evaluated using histology, immunostaining, biochemical analysis, and real-time polymerase chain reaction. Mitogen-activated protein kinase signaling pathways and p21 were compared in the DSCM and plastic-flask-expanded SDSCs with or without H2O2 treatment. We found that expansion on the DSCM upregulated antioxidative gene levels and chondrogenic potential in human SDSCs (hSDSCs), retarded the decrease in the cell number and the increase in apoptosis, and rendered SDSCs resistant to cell-cycle G1 arrest resulting from H2O2 treatment. Treatment with 0.05 mM H2O2 during cell expansion yielded pellets with increased chondrogenic differentiation; treatment in premature SDSC pellets showed that the DSCM-expanded cells had a robust resistance to H2O2-induced oxidative stress. Extracellular signal-regulated kinases 1 and 2 and p38 were positively involved in antioxidative and chondrogenic potential in SDSCs expanded on the DSCM in which p21 was downregulated. DSCM could be a promising cell expansion system to provide a large number of high-quality hSDSCs for cartilage regeneration in a harsh joint environment.
Figures

Similar articles
-
Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells.Tissue Eng Part A. 2013 May;19(9-10):1144-54. doi: 10.1089/ten.TEA.2012.0351. Epub 2013 Jan 15. Tissue Eng Part A. 2013. PMID: 23216161
-
Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix.Acta Biomater. 2015 Jul;20:39-50. doi: 10.1016/j.actbio.2015.04.001. Epub 2015 Apr 8. Acta Biomater. 2015. PMID: 25861949 Free PMC article.
-
Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration.Spine (Phila Pa 1976). 2012 Aug 15;37(18):1538-47. doi: 10.1097/BRS.0b013e31825150bf. Spine (Phila Pa 1976). 2012. PMID: 22391443
-
A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering.Eur Cell Mater. 2011 Nov 24;22:333-43; discussion 343. doi: 10.22203/ecm.v022a25. Eur Cell Mater. 2011. PMID: 22116651 Review.
-
Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration.Tissue Eng Part B Rev. 2012 Aug;18(4):301-11. doi: 10.1089/ten.TEB.2012.0002. Epub 2012 Apr 19. Tissue Eng Part B Rev. 2012. PMID: 22429320 Review.
Cited by
-
Melatonin reverses H2 O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway.J Pineal Res. 2015 Sep;59(2):190-205. doi: 10.1111/jpi.12250. Epub 2015 Jul 7. J Pineal Res. 2015. PMID: 25975679 Free PMC article.
-
Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources.Acta Biomater. 2018 Jul 1;74:56-73. doi: 10.1016/j.actbio.2018.04.048. Epub 2018 Apr 24. Acta Biomater. 2018. PMID: 29702288 Free PMC article. Review.
-
A Protocol to Prepare Decellularized Stem Cell Matrix for Rejuvenation of Cell Expansion and Cartilage Regeneration.Methods Mol Biol. 2018;1577:147-154. doi: 10.1007/7651_2017_27. Methods Mol Biol. 2018. PMID: 28451995 Free PMC article.
-
Differential Effects of the Antioxidants N-Acetylcysteine and Pyrrolidine Dithiocarbamate on Mesenchymal Stem Cell Chondrogenesis.Cell Mol Bioeng. 2019 Jan 18;12(2):153-163. doi: 10.1007/s12195-019-00566-3. eCollection 2019 Apr. Cell Mol Bioeng. 2019. PMID: 31719906 Free PMC article.
-
Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration.Stem Cell Rev Rep. 2014 Oct;10(5):686-96. doi: 10.1007/s12015-014-9526-z. Stem Cell Rev Rep. 2014. PMID: 24869958 Review.
References
-
- Fitzpatrick K. Tokish JM. A military perspective to articular cartilage defects. J Knee Surg. 2011;24:159–166. - PubMed
-
- John T. Stahel PF. Morgan SJ. Schulze-Tanzil G. Impact of the complement cascade on posttraumatic cartilage inflammation and degradation. Histol Histopathol. 2007;22:781–790. - PubMed
-
- Dhalla NS. Golfman L. Takeda S. Takeda N. Nagano M. Evidence for the role of oxidative stress in acute ischemic heart disease: a brief review. Can J Cardiol. 1999;15:587–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

