Hepatitis B virus X protein targets Bcl-2 proteins to increase intracellular calcium, required for virus replication and cell death induction
- PMID: 23091012
- PMCID: PMC3494919
- DOI: 10.1073/pnas.1204668109
Hepatitis B virus X protein targets Bcl-2 proteins to increase intracellular calcium, required for virus replication and cell death induction
Abstract
Infection with the hepatitis B virus (HBV) promotes the development of hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) and is a leading cause of morbidity and mortality worldwide. HBV X protein (HBx) is an important effector for HBV pathogenesis, but its cellular targets and acting mechanisms remain elusive. We show here that HBx interacts with the anti-apoptotic proteins Bcl-2 and Bcl-xL through a Bcl-2 homology 3 (BH3)-like motif in mammalian cells. Importantly, mutations in the BH3-like motif that prevent HBx binding to Bcl-2 and Bcl-xL abrogate cytosolic calcium elevation and cell death induced by HBx expression in hepatocytes and severely impair HBV viral replication, which can be substantially rescued by restoring cytosolic calcium. These results suggest that HBx binding to Bcl-2 family members and subsequent elevation of cytosolic calcium are important for HBV viral replication. Consistently, RNAi knockdown of Bcl-2 or Bcl-xL results in reduced calcium elevation by HBx and decreased viral replication in hepatocytes. Our results suggest that HBx targets Bcl-2 proteins through its BH3-like motif to promote cytosolic calcium elevation, cell death, and viral replication during HBV pathogenesis, which presents an excellent therapeutic intervention point in treating patients with chronic HBV.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

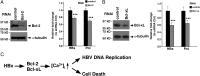
Similar articles
-
Structural and functional analyses of hepatitis B virus X protein BH3-like domain and Bcl-xL interaction.Nat Commun. 2019 Jul 19;10(1):3192. doi: 10.1038/s41467-019-11173-1. Nat Commun. 2019. PMID: 31324803 Free PMC article.
-
Hepatitis B virus X protein targets the Bcl-2 protein CED-9 to induce intracellular Ca2+ increase and cell death in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2012 Nov 6;109(45):18465-70. doi: 10.1073/pnas.1204652109. Epub 2012 Oct 22. Proc Natl Acad Sci U S A. 2012. PMID: 23091037 Free PMC article.
-
Chemical shift assignments of a fusion protein comprising the C-terminal-deleted hepatitis B virus X protein BH3-like motif peptide and Bcl-xL.Biomol NMR Assign. 2022 Oct;16(2):357-361. doi: 10.1007/s12104-022-10104-4. Epub 2022 Aug 31. Biomol NMR Assign. 2022. PMID: 36044106
-
[Role of HBx in hepatocellular carcinoma development].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010 May;39(3):333-8. doi: 10.3785/j.issn.1008-9292.2010.03.020. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 20544999 Review. Chinese.
-
The Epigenetic Modulation of Cancer and Immune Pathways in Hepatitis B Virus-Associated Hepatocellular Carcinoma: The Influence of HBx and miRNA Dysregulation.Front Immunol. 2021 Apr 29;12:661204. doi: 10.3389/fimmu.2021.661204. eCollection 2021. Front Immunol. 2021. PMID: 33995383 Free PMC article. Review.
Cited by
-
Hepatitis B virus X protein enhances Myc stability by inhibiting SCF(Skp2) ubiquitin E3 ligase-mediated Myc ubiquitination and contributes to oncogenesis.Oncogene. 2016 Apr 7;35(14):1857-67. doi: 10.1038/onc.2015.251. Epub 2015 Jul 13. Oncogene. 2016. PMID: 26165841
-
Structural and functional analyses of hepatitis B virus X protein BH3-like domain and Bcl-xL interaction.Nat Commun. 2019 Jul 19;10(1):3192. doi: 10.1038/s41467-019-11173-1. Nat Commun. 2019. PMID: 31324803 Free PMC article.
-
Immunoinformatics and Evaluation of Peptide Vaccines Derived from Global Hepatitis B Viral HBx and HBc Proteins Critical for Covalently Closed Circular DNA Integrity.Microorganisms. 2023 Nov 21;11(12):2826. doi: 10.3390/microorganisms11122826. Microorganisms. 2023. PMID: 38137971 Free PMC article.
-
Canonical and Divergent N-Terminal HBx Isoform Proteins Unveiled: Characteristics and Roles during HBV Replication.Biomedicines. 2021 Nov 16;9(11):1701. doi: 10.3390/biomedicines9111701. Biomedicines. 2021. PMID: 34829930 Free PMC article.
-
Anticancer compound ABT-263 accelerates apoptosis in virus-infected cells and imbalances cytokine production and lowers survival rates of infected mice.Cell Death Dis. 2013 Jul 25;4(7):e742. doi: 10.1038/cddis.2013.267. Cell Death Dis. 2013. PMID: 23887633 Free PMC article.
References
-
- Kremsdorf D, Soussan P, Paterlini-Brechot P, Brechot C. Hepatitis B virus-related hepatocellular carcinoma: Paradigms for viral-related human carcinogenesis. Oncogene. 2006;25:3823–3833. - PubMed
-
- Ganem D, Schneider RJ. The molecular biology of the hepatitis B viruses. In: Knipe DM, et al., editors. Fields Virology. 4th Ed. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2923–2970.
-
- Terradillos O, et al. The hepatitis B virus X protein abrogates Bcl-2-mediated protection against Fas apoptosis in the liver. Oncogene. 2002;21:377–386. - PubMed
-
- Shirakata Y, Koike K. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J Biol Chem. 2003;278:22071–22078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

