CD133-expressing thyroid cancer cells are undifferentiated, radioresistant and survive radioiodide therapy
- PMID: 23081821
- PMCID: PMC3510415
- DOI: 10.1007/s00259-012-2242-5
CD133-expressing thyroid cancer cells are undifferentiated, radioresistant and survive radioiodide therapy
Abstract
Purpose: (131)I therapy is regularly used following surgery as a part of thyroid cancer management. Despite an overall relatively good prognosis, recurrent or metastatic thyroid cancer is not rare. CD133-expressing cells have been shown to mark thyroid cancer stem cells that possess the characteristics of stem cells and have the ability to initiate tumours. However, no studies have addressed the influence of CD133-expressing cells on radioiodide therapy of the thyroid cancer. The aim of this study was to investigate whether CD133(+) cells contribute to the radioresistance of thyroid cancer and thus potentiate future recurrence and metastasis.
Methods: Thyroid cancer cell lines were analysed for CD133 expression, radiosensitivity and gene expression.
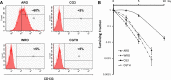
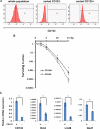
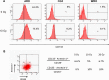
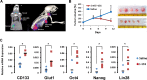
Results: The anaplastic thyroid cancer cell line ARO showed a higher percentage of CD133(+) cells and higher radioresistance. After γ-irradiation of the cells, the CD133(+) population was enriched due to the higher apoptotic rate of CD133(-) cells. In vivo (131)I treatment of ARO tumour resulted in an elevated expression of CD133, Oct4, Nanog, Lin28 and Glut1 genes. After isolation, CD133(+) cells exhibited higher radioresistance and higher expression of Oct4, Nanog, Sox2, Lin28 and Glut1 in the cell line or primarily cultured papillary thyroid cancer cells, and lower expression of various thyroid-specific genes, namely NIS, Tg, TPO, TSHR, TTF1 and Pax8.
Conclusion: This study demonstrates the existence of CD133-expressing thyroid cancer cells which show a higher radioresistance and are in an undifferentiated status. These cells possess a greater potential to survive radiotherapy and may contribute to the recurrence of thyroid cancer. A future therapeutic approach for radioresistant thyroid cancer may focus on the selective eradication of CD133(+) cells.
Figures

Similar articles
-
Targeting signal transducer and activator of transcription 3 pathway by cucurbitacin I diminishes self-renewing and radiochemoresistant abilities in thyroid cancer-derived CD133+ cells.J Pharmacol Exp Ther. 2012 May;341(2):410-23. doi: 10.1124/jpet.111.188730. Epub 2012 Feb 10. J Pharmacol Exp Ther. 2012. PMID: 22328572
-
CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma.Cancer Lett. 2012 Feb 28;315(2):129-37. doi: 10.1016/j.canlet.2011.10.012. Epub 2011 Oct 23. Cancer Lett. 2012. PMID: 22079466
-
Hypoxia promotes radioresistance of CD133-positive Hep-2 human laryngeal squamous carcinoma cells in vitro.Int J Oncol. 2013 Jul;43(1):131-40. doi: 10.3892/ijo.2013.1929. Epub 2013 May 2. Int J Oncol. 2013. PMID: 23652853
-
Glioma stem cells promote radioresistance by preferential activation of the DNA damage response.Nature. 2006 Dec 7;444(7120):756-60. doi: 10.1038/nature05236. Epub 2006 Oct 18. Nature. 2006. PMID: 17051156
-
Targeting CD133 antigen in cancer.Expert Opin Ther Targets. 2009 Jul;13(7):823-37. doi: 10.1517/14728220903005616. Expert Opin Ther Targets. 2009. PMID: 19530986 Review.
Cited by
-
Expression of Gp78/Autocrine Motility Factor Receptor and Endocytosis of Autocrine Motility Factor in Human Thyroid Cancer Cells.Cureus. 2019 Jun 17;11(6):e4928. doi: 10.7759/cureus.4928. Cureus. 2019. PMID: 31431834 Free PMC article.
-
In vivo 5FU-exposed human medullary thyroid carcinoma cells contain a chemoresistant CD133+ tumor-initiating cell subset.Thyroid. 2014 Mar;24(3):520-32. doi: 10.1089/thy.2013.0277. Epub 2013 Dec 13. Thyroid. 2014. PMID: 24073856 Free PMC article.
-
Stemness in human thyroid cancers and derived cell lines: the role of asymmetrically dividing cancer stem cells resistant to chemotherapy.J Clin Endocrinol Metab. 2014 Mar;99(3):E400-9. doi: 10.1210/jc.2013-3545. Epub 2014 Feb 25. J Clin Endocrinol Metab. 2014. PMID: 24823711 Free PMC article.
-
Thyroid malignant neoplasm-associated biomarkers as targets for oncolytic virotherapy.Oncolytic Virother. 2016 Jun 21;5:35-43. doi: 10.2147/OV.S99856. eCollection 2016. Oncolytic Virother. 2016. PMID: 27579295 Free PMC article. Review.
-
Thyroid tumor-initiating cells: increasing evidence and opportunities for anticancer therapy (review).Oncol Rep. 2014 Mar;31(3):1035-42. doi: 10.3892/or.2014.2978. Epub 2014 Jan 14. Oncol Rep. 2014. PMID: 24424445 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

