The peptide specificity of the endogenous T follicular helper cell repertoire generated after protein immunization
- PMID: 23077537
- PMCID: PMC3471970
- DOI: 10.1371/journal.pone.0046952
The peptide specificity of the endogenous T follicular helper cell repertoire generated after protein immunization
Abstract
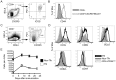
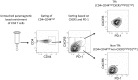
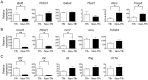
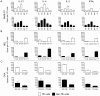
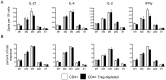
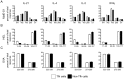
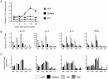
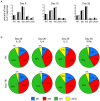
T follicular helper (Tfh) cells potentiate high-affinity, class-switched antibody responses, the predominant correlate of protection from vaccines. Despite intense interest in understanding both the generation and effector functions of this lineage, little is known about the epitope specificity of Tfh cells generated during polyclonal responses. To date, studies of peptide-specific Tfh cells have relied on either the transfer of TcR transgenic cells or use of peptide:MHC class II tetramers and antibodies to stain TcR and follow limited peptide specificities. In order to comprehensively evaluate polyclonal responses generated from the natural endogenous TcR repertoire, we developed a sorting strategy to separate Tfh cells from non-Tfh cells and found that their epitope-specific responses could be tracked with cytokine-specific ELISPOT assays. The immunodominance hierarchies of Tfh and non-Tfh cells generated in response to immunization with several unrelated protein antigens were remarkably similar. Additionally, increasing the kinetic stability of peptide-MHC class II complexes enhanced the priming of both Tfh and conventional CD4 T cells. These findings may provide us with a strategy to rationally and selectively modulate epitope-specific Tfh responses. By understanding the parameters that control epitope-specific priming, vaccines may be tailored to enhance or focus Tfh responses to facilitate optimal B cell responses.
Conflict of interest statement
Figures
Similar articles
-
CD4 T cell epitope specificity determines follicular versus non-follicular helper differentiation in the polyclonal response to influenza infection or vaccination.Sci Rep. 2016 Jun 22;6:28287. doi: 10.1038/srep28287. Sci Rep. 2016. PMID: 27329272 Free PMC article.
-
T follicular helper and T follicular regulatory cells have different TCR specificity.Nat Commun. 2017 Apr 21;8:15067. doi: 10.1038/ncomms15067. Nat Commun. 2017. PMID: 28429709 Free PMC article.
-
Strength of tonic T cell receptor signaling instructs T follicular helper cell-fate decisions.Nat Immunol. 2020 Nov;21(11):1384-1396. doi: 10.1038/s41590-020-0781-7. Epub 2020 Sep 28. Nat Immunol. 2020. PMID: 32989327 Free PMC article.
-
Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses.Front Immunol. 2018 Oct 22;9:2412. doi: 10.3389/fimmu.2018.02412. eCollection 2018. Front Immunol. 2018. PMID: 30405612 Free PMC article. Review.
-
Signals that drive T follicular helper cell formation.Immunology. 2017 Oct;152(2):185-194. doi: 10.1111/imm.12778. Epub 2017 Jul 17. Immunology. 2017. PMID: 28628194 Free PMC article. Review.
Cited by
-
CD4 T cell epitope specificity determines follicular versus non-follicular helper differentiation in the polyclonal response to influenza infection or vaccination.Sci Rep. 2016 Jun 22;6:28287. doi: 10.1038/srep28287. Sci Rep. 2016. PMID: 27329272 Free PMC article.
-
How Germinal Centers Evolve Broadly Neutralizing Antibodies: the Breadth of the Follicular Helper T Cell Response.J Virol. 2017 Oct 27;91(22):e00983-17. doi: 10.1128/JVI.00983-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28878083 Free PMC article.
-
Follicular Helper T Cells in Systemic Lupus Erythematosus.Front Immunol. 2018 Aug 3;9:1793. doi: 10.3389/fimmu.2018.01793. eCollection 2018. Front Immunol. 2018. PMID: 30123218 Free PMC article. Review.
-
Narrowing the Gap: Preserving Repertoire Diversity Despite Clonal Selection during the CD4 T Cell Response.Front Immunol. 2015 Aug 11;6:413. doi: 10.3389/fimmu.2015.00413. eCollection 2015. Front Immunol. 2015. PMID: 26322045 Free PMC article. Review.
-
H7N9 T-cell epitopes that mimic human sequences are less immunogenic and may induce Treg-mediated tolerance.Hum Vaccin Immunother. 2015;11(9):2241-52. doi: 10.1080/21645515.2015.1052197. Epub 2015 Jun 19. Hum Vaccin Immunother. 2015. PMID: 26090577 Free PMC article.
References
-
- Siegrist C (2008) Vaccine Immunology. In: Plotkin S, editor. Vaccines: Expert Consult. 5 ed. Philadelphia: Saunders Elsevier. pp. 17–36.
-
- Arnold CN, Campbell DJ, Lipp M, Butcher EC (2007) The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol 37: 100–109. - PubMed
-
- Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, et al. (2010) Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol 185: 4042–4052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

