Staphylococcus aureus proteins Sbi and Efb recruit human plasmin to degrade complement C3 and C3b
- PMID: 23071827
- PMCID: PMC3469469
- DOI: 10.1371/journal.pone.0047638
Staphylococcus aureus proteins Sbi and Efb recruit human plasmin to degrade complement C3 and C3b
Abstract
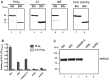
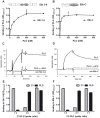
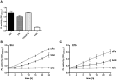
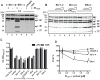
Upon host infection, the human pathogenic microbe Staphylococcus aureus (S. aureus) immediately faces innate immune reactions such as the activated complement system. Here, a novel innate immune evasion strategy of S. aureus is described. The staphylococcal proteins surface immunoglobulin-binding protein (Sbi) and extracellular fibrinogen-binding protein (Efb) bind C3/C3b simultaneously with plasminogen. Bound plasminogen is converted by bacterial activator staphylokinase or by host-specific urokinase-type plasminogen activator to plasmin, which in turn leads to degradation of complement C3 and C3b. Efb and to a lesser extend Sbi enhance plasmin cleavage of C3/C3b, an effect which is explained by a conformational change in C3/C3b induced by Sbi and Efb. Furthermore, bound plasmin also degrades C3a, which exerts anaphylatoxic and antimicrobial activities. Thus, S. aureus Sbi and Efb comprise platforms to recruit plasmin(ogen) together with C3 and its activation product C3b for efficient degradation of these complement components in the local microbial environment and to protect S. aureus from host innate immune reactions.
Conflict of interest statement
Figures

Similar articles
-
Allosteric inhibition of complement function by a staphylococcal immune evasion protein.Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17621-6. doi: 10.1073/pnas.1003750107. Epub 2010 Sep 27. Proc Natl Acad Sci U S A. 2010. PMID: 20876141 Free PMC article.
-
Plasmin cleaves fibrinogen and the human complement proteins C3b and C5 in the presence of Leptospira interrogans proteins: A new role of LigA and LigB in invasion and complement immune evasion.Immunobiology. 2016 May;221(5):679-89. doi: 10.1016/j.imbio.2016.01.001. Epub 2016 Jan 7. Immunobiology. 2016. PMID: 26822552
-
Mutational analyses reveal that the staphylococcal immune evasion molecule Sbi and complement receptor 2 (CR2) share overlapping contact residues on C3d: implications for the controversy regarding the CR2/C3d cocrystal structure.J Immunol. 2010 Feb 15;184(4):1946-55. doi: 10.4049/jimmunol.0902919. Epub 2010 Jan 18. J Immunol. 2010. PMID: 20083651
-
Advances in understanding the structure, function, and mechanism of the SCIN and Efb families of Staphylococcal immune evasion proteins.Adv Exp Med Biol. 2012;946:113-33. doi: 10.1007/978-1-4614-0106-3_7. Adv Exp Med Biol. 2012. PMID: 21948365 Free PMC article. Review.
-
Structural insights on complement activation.FEBS J. 2015 Oct;282(20):3883-91. doi: 10.1111/febs.13399. Epub 2015 Aug 31. FEBS J. 2015. PMID: 26250513 Review.
Cited by
-
Molecular Interactions of Human Plasminogen with Fibronectin-binding Protein B (FnBPB), a Fibrinogen/Fibronectin-binding Protein from Staphylococcus aureus.J Biol Chem. 2016 Aug 26;291(35):18148-62. doi: 10.1074/jbc.M116.731125. Epub 2016 Jul 7. J Biol Chem. 2016. PMID: 27387503 Free PMC article.
-
A Novel Interaction between Complement Inhibitor C4b-binding Protein and Plasminogen That Enhances Plasminogen Activation.J Biol Chem. 2015 Jul 24;290(30):18333-42. doi: 10.1074/jbc.M114.619494. Epub 2015 Jun 11. J Biol Chem. 2015. PMID: 26067271 Free PMC article.
-
Roles of the Crp/Fnr Family Regulator ArcR in the Hemolysis and Biofilm of Staphylococcus aureus.Microorganisms. 2023 Jun 25;11(7):1656. doi: 10.3390/microorganisms11071656. Microorganisms. 2023. PMID: 37512829 Free PMC article.
-
Fibrinogen Activates the Capture of Human Plasminogen by Staphylococcal Fibronectin-Binding Proteins.mBio. 2017 Sep 5;8(5):e01067-17. doi: 10.1128/mBio.01067-17. mBio. 2017. PMID: 28874469 Free PMC article.
-
In-silico prediction of drug targets, biological activities, signal pathways and regulating networks of dioscin based on bioinformatics.BMC Complement Altern Med. 2015 Mar 5;15:41. doi: 10.1186/s12906-015-0579-6. BMC Complement Altern Med. 2015. PMID: 25879470 Free PMC article.
References
-
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520–532. - PubMed
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, et al. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298: 1763–1771. - PubMed
-
- Walport MJ (2001) Complement. First of two parts. N Engl J Med 344: 1058–1066. - PubMed
-
- Zipfel PF, Skerka C (2009) Complement regulators and inhibitory proteins. Nature Reviews Immunology 9: 729–740. - PubMed
-
- Walport MJ (2001) Complement. Second of two parts. N Engl J Med 344: 1140–1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

