Multicenter phase II study of plitidepsin in patients with relapsed/refractory non-Hodgkin's lymphoma
- PMID: 23065525
- PMCID: PMC3659946
- DOI: 10.3324/haematol.2012.069757
Multicenter phase II study of plitidepsin in patients with relapsed/refractory non-Hodgkin's lymphoma
Abstract
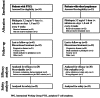
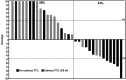
This phase II clinical trial evaluated the efficacy, safety and pharmacokinetics of plitidepsin 3.2 mg/m(2) administered as a 1-hour intravenous infusion weekly on days 1, 8 and 15 every 4 weeks in 67 adult patients with relapsed/refractory aggressive non-Hodgkin's lymphoma. Patients were divided into two cohorts: those with non-cutaneous peripheral T-cell lymphoma (n=34) and those with other lymphomas (n=33). Efficacy was evaluated using the International Working Group criteria (1999). Of the 29 evaluable patients with non-cutaneous peripheral T-cell lymphoma, six had a response (overall response rate 20.7%; 95% confidence interval, 8.0%-39.7%), including two complete responses and four partial responses. No responses occurred in the 30 evaluable patients with other lymphomas (including 27 B-cell lymphomas). The most common plitidepsin-related adverse events were nausea, fatigue and myalgia (grade 3 in <10% of cases). Severe laboratory abnormalities (lymphopenia, anemia, thrombocytopenia, and increased levels of transaminase and creatine phosphokinase) were transient and easily managed by plitidepsin dose adjustments. The pharmacokinetic profile did not differ from that previously reported in patients with solid tumors. In conclusion, plitidepsin monotherapy has clinical activity in relapsed/refractory T-cell lymphomas. Combinations of plitidepsin with other chemotherapeutic drugs deserve further evaluation in patients with non-cutaneous peripheral T-cell lymphoma. (clinicaltrials.gov identifier: NCT00884286).
Figures
Similar articles
-
Plitidepsin: design, development, and potential place in therapy.Drug Des Devel Ther. 2017 Jan 19;11:253-264. doi: 10.2147/DDDT.S94165. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28176904 Free PMC article. Review.
-
Phase II clinical and pharmacokinetic study of plitidepsin 3-hour infusion every two weeks alone or with dexamethasone in relapsed and refractory multiple myeloma.Clin Cancer Res. 2010 Jun 15;16(12):3260-9. doi: 10.1158/1078-0432.CCR-10-0469. Epub 2010 Jun 8. Clin Cancer Res. 2010. PMID: 20530693 Clinical Trial.
-
Phase I clinical and pharmacokinetic study of plitidepsin as a 1-hour weekly intravenous infusion in patients with advanced solid tumors.Clin Cancer Res. 2008 May 15;14(10):3105-12. doi: 10.1158/1078-0432.CCR-07-1652. Clin Cancer Res. 2008. PMID: 18483378 Clinical Trial.
-
A Phase I Dose-Escalation Study of Clofarabine in Patients with Relapsed or Refractory Low-Grade or Intermediate-Grade B-Cell or T-Cell Lymphoma.Oncologist. 2018 Apr;23(4):397-e30. doi: 10.1634/theoncologist.2017-0658. Epub 2018 Feb 7. Oncologist. 2018. PMID: 29438091 Free PMC article. Clinical Trial.
-
Treatment of relapsed aggressive lymphomas: regimens with and without high-dose therapy and stem cell rescue.Cancer Chemother Pharmacol. 2002 May;49 Suppl 1:S13-20. doi: 10.1007/s00280-002-0447-1. Epub 2002 Apr 12. Cancer Chemother Pharmacol. 2002. PMID: 12042984 Review.
Cited by
-
Marine Natural Products in Clinical Use.Mar Drugs. 2022 Aug 18;20(8):528. doi: 10.3390/md20080528. Mar Drugs. 2022. PMID: 36005531 Free PMC article. Review.
-
Self-Assembling Hybrid Linear-Dendritic Block Copolymers: The Design of Nano-Carriers for Lipophilic Antitumoral Drugs.Nanomaterials (Basel). 2019 Jan 29;9(2):161. doi: 10.3390/nano9020161. Nanomaterials (Basel). 2019. PMID: 30699915 Free PMC article.
-
Plitidepsin: design, development, and potential place in therapy.Drug Des Devel Ther. 2017 Jan 19;11:253-264. doi: 10.2147/DDDT.S94165. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28176904 Free PMC article. Review.
-
Marine-derived angiogenesis inhibitors for cancer therapy.Mar Drugs. 2013 Mar 15;11(3):903-33. doi: 10.3390/md11030903. Mar Drugs. 2013. PMID: 23502698 Free PMC article. Review.
-
Plitidepsin: Mechanisms and Clinical Profile of a Promising Antiviral Agent against COVID-19.J Pers Med. 2021 Jul 16;11(7):668. doi: 10.3390/jpm11070668. J Pers Med. 2021. PMID: 34357135 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300 - PubMed
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765–81 - PubMed
-
- Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117–26 - PubMed
-
- Rudiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 2002;13(1):140–9 - PubMed
-
- Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–67 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

