Serine is a natural ligand and allosteric activator of pyruvate kinase M2
- PMID: 23064226
- PMCID: PMC3894725
- DOI: 10.1038/nature11540
Serine is a natural ligand and allosteric activator of pyruvate kinase M2
Erratum in
- Nature. 2013 Apr 18;496(7445):386
Abstract
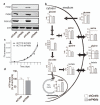
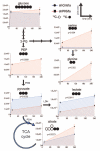
Cancer cells exhibit several unique metabolic phenotypes that are critical for cell growth and proliferation. Specifically, they overexpress the M2 isoform of the tightly regulated enzyme pyruvate kinase (PKM2), which controls glycolytic flux, and are highly dependent on de novo biosynthesis of serine and glycine. Here we describe a new rheostat-like mechanistic relationship between PKM2 activity and serine biosynthesis. We show that serine can bind to and activate human PKM2, and that PKM2 activity in cells is reduced in response to serine deprivation. This reduction in PKM2 activity shifts cells to a fuel-efficient mode in which more pyruvate is diverted to the mitochondria and more glucose-derived carbon is channelled into serine biosynthesis to support cell proliferation.
Figures


Similar articles
-
Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation.Proc Natl Acad Sci U S A. 2012 May 1;109(18):6904-9. doi: 10.1073/pnas.1204176109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509023 Free PMC article.
-
Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis.Nat Chem Biol. 2012 Oct;8(10):839-47. doi: 10.1038/nchembio.1060. Nat Chem Biol. 2012. PMID: 22922757 Free PMC article.
-
Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2.Mol Cancer. 2013 Jul 9;12:72. doi: 10.1186/1476-4598-12-72. Mol Cancer. 2013. PMID: 23837608 Free PMC article.
-
Pyruvate kinase: Function, regulation and role in cancer.Semin Cell Dev Biol. 2015 Jul;43:43-51. doi: 10.1016/j.semcdb.2015.08.004. Epub 2015 Aug 13. Semin Cell Dev Biol. 2015. PMID: 26277545 Free PMC article. Review.
-
Metabolic pathway alterations that support cell proliferation.Cold Spring Harb Symp Quant Biol. 2011;76:325-34. doi: 10.1101/sqb.2012.76.010900. Epub 2012 Jan 19. Cold Spring Harb Symp Quant Biol. 2011. PMID: 22262476 Review.
Cited by
-
CIP2A induces PKM2 tetramer formation and oxidative phosphorylation in non-small cell lung cancer.Cell Discov. 2024 Feb 6;10(1):13. doi: 10.1038/s41421-023-00633-0. Cell Discov. 2024. PMID: 38321019 Free PMC article.
-
Pyruvate Kinase M2: A Potential Target for Regulating Inflammation.Front Immunol. 2016 Apr 21;7:145. doi: 10.3389/fimmu.2016.00145. eCollection 2016. Front Immunol. 2016. PMID: 27148264 Free PMC article. Review.
-
Altered metabolite levels in cancer: implications for tumour biology and cancer therapy.Nat Rev Cancer. 2016 Nov;16(11):680-693. doi: 10.1038/nrc.2016.85. Epub 2016 Sep 23. Nat Rev Cancer. 2016. PMID: 27658530 Review.
-
The role of glycine in regulated cell death.Cell Mol Life Sci. 2016 Jun;73(11-12):2285-308. doi: 10.1007/s00018-016-2201-6. Epub 2016 Apr 11. Cell Mol Life Sci. 2016. PMID: 27066896 Free PMC article. Review.
-
Metabolic Phenotypes, Dependencies, and Adaptation in Lung Cancer.Cold Spring Harb Perspect Med. 2021 Nov 1;11(11):a037838. doi: 10.1101/cshperspect.a037838. Cold Spring Harb Perspect Med. 2021. PMID: 34127512 Free PMC article. Review.
References
-
- Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–277. doi:nrc2817 [pii] 10.1038/nrc2817. - PubMed
-
- Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi:nature06734 [pii] 10.1038/nature06734. - PubMed
-
- Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi:S0888-7543(04)00227-7 [pii] 10.1016/j.ygeno.2004.08.010. - PubMed
-
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi:S1044-579X(05)00026-X [pii] 10.1016/j.semcancer.2005.04.009. - PubMed
Methods References
-
- Luo B, Groenke K, Takors R, Wandrey C, Oldiges M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. J Chromatogr A. 2007;1147:153–164. doi:S0021-9673(07)00290-7 [pii] 10.1016/j.chroma.2007.02.034. - PubMed
-
- Salituro FG, Saunders JO. Therapeutic compositions and related methods of use. WO/2010/118063 patent. 2010:52.
-
- Collaborative Computational Project Number 4. Acta Cryst. 1994;D50:760–763. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

