Molecular machines governing exocytosis of synaptic vesicles
- PMID: 23060190
- PMCID: PMC4461657
- DOI: 10.1038/nature11320
Molecular machines governing exocytosis of synaptic vesicles
Abstract
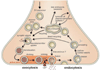
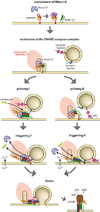
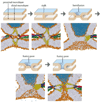
Calcium-dependent exocytosis of synaptic vesicles mediates the release of neurotransmitters. Important proteins in this process have been identified such as the SNAREs, synaptotagmins, complexins, Munc18 and Munc13. Structural and functional studies have yielded a wealth of information about the physiological role of these proteins. However, it has been surprisingly difficult to arrive at a unified picture of the molecular sequence of events from vesicle docking to calcium-triggered membrane fusion. Using mainly a biochemical and biophysical perspective, we briefly survey the molecular mechanisms in an attempt to functionally integrate the key proteins into the emerging picture of the neuronal fusion machine.
Figures

Similar articles
-
Munc13-1 MUN domain and Munc18-1 cooperatively chaperone SNARE assembly through a tetrameric complex.Proc Natl Acad Sci U S A. 2020 Jan 14;117(2):1036-1041. doi: 10.1073/pnas.1914361117. Epub 2019 Dec 30. Proc Natl Acad Sci U S A. 2020. PMID: 31888993 Free PMC article.
-
Inside insight to membrane fusion.Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11729-30. doi: 10.1073/pnas.1108770108. Epub 2011 Jul 7. Proc Natl Acad Sci U S A. 2011. PMID: 21737746 Free PMC article. No abstract available.
-
Synaptic vesicle exocytosis.Cold Spring Harb Perspect Biol. 2011 Dec 1;3(12):a005637. doi: 10.1101/cshperspect.a005637. Cold Spring Harb Perspect Biol. 2011. PMID: 22026965 Free PMC article. Review.
-
Mechanisms of synaptic vesicle exocytosis.Annu Rev Cell Dev Biol. 2000;16:19-49. doi: 10.1146/annurev.cellbio.16.1.19. Annu Rev Cell Dev Biol. 2000. PMID: 11031229 Review.
-
Beyond the MUN domain, Munc13 controls priming and depriming of synaptic vesicles.Cell Rep. 2024 May 28;43(5):114026. doi: 10.1016/j.celrep.2024.114026. Epub 2024 May 21. Cell Rep. 2024. PMID: 38809756 Free PMC article.
Cited by
-
Protein-phospholipid interactions in nonclassical protein secretion: problem and methods of study.Int J Mol Sci. 2013 Feb 8;14(2):3734-72. doi: 10.3390/ijms14023734. Int J Mol Sci. 2013. PMID: 23396106 Free PMC article.
-
Revisit the Correlation between the Elastic Mechanics and Fusion of Lipid Membranes.Sci Rep. 2016 Aug 18;6:31470. doi: 10.1038/srep31470. Sci Rep. 2016. PMID: 27534263 Free PMC article.
-
Microsporidia dressing up: the spore polaroplast transport through the polar tube and transformation into the sporoplasm membrane.mBio. 2024 Feb 14;15(2):e0274923. doi: 10.1128/mbio.02749-23. Epub 2024 Jan 9. mBio. 2024. PMID: 38193684 Free PMC article.
-
Regulation of membrane trafficking by signalling on endosomal and lysosomal membranes.J Physiol. 2013 Sep 15;591(18):4389-401. doi: 10.1113/jphysiol.2013.258301. Epub 2013 Jul 22. J Physiol. 2013. PMID: 23878375 Free PMC article. Review.
-
Early life seizures and epileptic spasms in STXBP1-related disorders.Epilepsia. 2024 Mar;65(3):805-816. doi: 10.1111/epi.17886. Epub 2024 Jan 27. Epilepsia. 2024. PMID: 38279907
References
-
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

