Dynamic reorganization of metabolic enzymes into intracellular bodies
- PMID: 23057741
- PMCID: PMC4089986
- DOI: 10.1146/annurev-cellbio-101011-155841
Dynamic reorganization of metabolic enzymes into intracellular bodies
Abstract
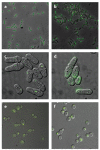
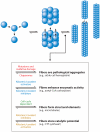
Both focused and large-scale cell biological and biochemical studies have revealed that hundreds of metabolic enzymes across diverse organisms form large intracellular bodies. These proteinaceous bodies range in form from fibers and intracellular foci--such as those formed by enzymes of nitrogen and carbon utilization and of nucleotide biosynthesis--to high-density packings inside bacterial microcompartments and eukaryotic microbodies. Although many enzymes clearly form functional mega-assemblies, it is not yet clear for many recently discovered cases whether they represent functional entities, storage bodies, or aggregates. In this article, we survey intracellular protein bodies formed by metabolic enzymes, asking when and why such bodies form and what their formation implies for the functionality--and dysfunctionality--of the enzymes that comprise them. The panoply of intracellular protein bodies also raises interesting questions regarding their evolution and maintenance within cells. We speculate on models for how such structures form in the first place and why they may be inevitable.
Figures





Similar articles
-
Cellular stress leads to the formation of membraneless stress assemblies in eukaryotic cells.Traffic. 2019 Sep;20(9):623-638. doi: 10.1111/tra.12669. Epub 2019 Jul 30. Traffic. 2019. PMID: 31152627 Free PMC article. Review.
-
Protein kinases are associated with multiple, distinct cytoplasmic granules in quiescent yeast cells.Genetics. 2014 Dec;198(4):1495-512. doi: 10.1534/genetics.114.172031. Epub 2014 Oct 23. Genetics. 2014. PMID: 25342717 Free PMC article.
-
Inclusion body anatomy and functioning of chaperone-mediated in vivo inclusion body disassembly during high-level recombinant protein production in Escherichia coli.J Biotechnol. 2007 Jan 1;127(2):244-57. doi: 10.1016/j.jbiotec.2006.07.004. Epub 2006 Jul 16. J Biotechnol. 2007. PMID: 16945443
-
Yeast peroxisomes: How are they formed and how do they grow?Int J Biochem Cell Biol. 2018 Dec;105:24-34. doi: 10.1016/j.biocel.2018.09.019. Epub 2018 Sep 27. Int J Biochem Cell Biol. 2018. PMID: 30268746 Review.
-
Patterns of indirect protein interactions suggest a spatial organization to metabolism.Mol Biosyst. 2011 Nov;7(11):3056-64. doi: 10.1039/c1mb05168g. Epub 2011 Aug 31. Mol Biosyst. 2011. PMID: 21881679
Cited by
-
Rubisco forms a lattice inside alpha-carboxysomes.Nat Commun. 2022 Aug 18;13(1):4863. doi: 10.1038/s41467-022-32584-7. Nat Commun. 2022. PMID: 35982043 Free PMC article.
-
Anti-rods/rings autoantibody generation in hepatitis C patients during interferon-α/ribavirin therapy.World J Gastroenterol. 2016 Feb 14;22(6):1966-74. doi: 10.3748/wjg.v22.i6.1966. World J Gastroenterol. 2016. PMID: 26877604 Free PMC article. Review.
-
Snf1/AMPK promotes the formation of Kog1/Raptor-bodies to increase the activation threshold of TORC1 in budding yeast.Elife. 2015 Oct 6;4:e09181. doi: 10.7554/eLife.09181. Elife. 2015. PMID: 26439012 Free PMC article.
-
Glycolytic Enzymes Coalesce in G Bodies under Hypoxic Stress.Cell Rep. 2017 Jul 25;20(4):895-908. doi: 10.1016/j.celrep.2017.06.082. Cell Rep. 2017. PMID: 28746874 Free PMC article.
-
Mechanism of Filamentation-Induced Allosteric Activation of the SgrAI Endonuclease.Structure. 2019 Oct 1;27(10):1497-1507.e3. doi: 10.1016/j.str.2019.08.001. Epub 2019 Aug 22. Structure. 2019. PMID: 31447289 Free PMC article.
References
-
- An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320(5872):103–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

