Unique C2V3 sequence in HIV-1 envelope obtained from broadly neutralizing plasma of a slow progressing patient conferred enhanced virus neutralization
- PMID: 23056416
- PMCID: PMC3463516
- DOI: 10.1371/journal.pone.0046713
Unique C2V3 sequence in HIV-1 envelope obtained from broadly neutralizing plasma of a slow progressing patient conferred enhanced virus neutralization
Abstract
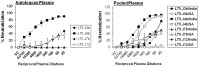
Broadly neutralizing antibodies to HIV-1 usually develops in chronic infections. Here, we examined the basis of enhanced sensitivity of an env clone amplified from cross neutralizing plasma of an antiretroviral naïve chronically infected Indian patient (ID50 >600-fold higher compared to other autologous env clones). The enhanced autologous neutralization of pseudotyped viruses expressing the sensitive envelope (Env) was associated with increased sensitivity to reagents and monoclonal antibodies targeting distinct sites in Env. Chimeric viruses constructed by swapping fragments of sensitive Env into resistant Env backbone revealed that the presence of unique residues within C2V3 region of gp120 governed increased neutralization. The enhanced virus neutralization was also associated with low CD4 dependence as well as increased binding of Env trimers to IgG1b12 and CD4-IgG2 and was independent of gp120 shedding. Our data highlighted vulnerabilities in the Env obtained from cross neutralizing plasma associated with the exposure of discontinuous neutralizing epitopes and enhanced autologous neutralization. Such information may aid in Env-based vaccine immunogen design.
Conflict of interest statement
Figures





Similar articles
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
Variations in autologous neutralization and CD4 dependence of b12 resistant HIV-1 clade C env clones obtained at different time points from antiretroviral naïve Indian patients with recent infection.Retrovirology. 2010 Sep 22;7:76. doi: 10.1186/1742-4690-7-76. Retrovirology. 2010. PMID: 20860805 Free PMC article.
-
Association of mutations in V3/C3 domain with enhanced sensitivity of HIV-1 clade C primary envelopes to autologous broadly neutralizing plasma antibodies.Retrovirology. 2016 Jun 15;13(1):41. doi: 10.1186/s12977-016-0273-x. Retrovirology. 2016. PMID: 27307004 Free PMC article.
-
A variable region 3 (V3) mutation determines a global neutralization phenotype and CD4-independent infectivity of a human immunodeficiency virus type 1 envelope associated with a broadly cross-reactive, primary virus-neutralizing antibody response.J Virol. 2002 Jan;76(2):644-55. doi: 10.1128/jvi.76.2.644-655.2002. J Virol. 2002. PMID: 11752155 Free PMC article.
-
Identification of Novel Structural Determinants in MW965 Env That Regulate the Neutralization Phenotype and Conformational Masking Potential of Primary HIV-1 Isolates.J Virol. 2018 Feb 12;92(5):e01779-17. doi: 10.1128/JVI.01779-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237828 Free PMC article.
Cited by
-
Characterization of a large cluster of HIV-1 A1 infections detected in Portugal and connected to several Western European countries.Sci Rep. 2019 May 10;9(1):7223. doi: 10.1038/s41598-019-43420-2. Sci Rep. 2019. PMID: 31076722 Free PMC article.
-
Characterization of a stable HIV-1 B/C recombinant, soluble, and trimeric envelope glycoprotein (Env) highly resistant to CD4-induced conformational changes.J Biol Chem. 2017 Sep 22;292(38):15849-15858. doi: 10.1074/jbc.M117.803056. Epub 2017 Jul 25. J Biol Chem. 2017. PMID: 28743743 Free PMC article.
-
Inspecting the interaction between human immunodeficiency virus and the immune system through genetic turnover.Philos Trans R Soc Lond B Biol Sci. 2023 May 22;378(1877):20220056. doi: 10.1098/rstb.2022.0056. Epub 2023 Apr 3. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37004725 Free PMC article.
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
References
-
- Beirnaert E, Nyambi P, Willems B, Heyndrickx L, Colebunders R, et al. (2000) Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (env clade A-H) and group O primary HIV-1 isolates. J Med Virol 62: 14–24. - PubMed
-
- Simek MD, Rida W, Priddy FH, Pung P, Carrow E, et al. (2009) Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 83: 7337–7348. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

