Increased synapse formation obtained by T cell epitopes containing a CxxC motif in flanking residues convert CD4+ T cells into cytolytic effectors
- PMID: 23056200
- PMCID: PMC3467281
- DOI: 10.1371/journal.pone.0045366
Increased synapse formation obtained by T cell epitopes containing a CxxC motif in flanking residues convert CD4+ T cells into cytolytic effectors
Abstract
The nature of MHC class II-binding epitopes not only determines the specificity of T cell responses, but may also alter effector cell functions. Cytolytic CD4+ T cells have been observed primarily in anti-viral responses, but very little is known about the conditions under which they can be elicited. Their potential as regulators of immune responses, however, deserves investigations. We describe here that inclusion of a thiol-disulfide oxidoreductase motif within flanking residues of class II-restricted epitopes results, both in vitro and in vivo, in elicitation of antigen-specific cytolytic CD4+ T cells through increased synapse formation. We show that both naïve and polarized CD4+ T cells, including Th17 cells, can be converted by cognate recognition of such modified epitopes. Cytolytic CD4+ T cells induce apoptosis on APCs by Fas-FasL interaction. These findings potentially open the way towards a novel form of antigen-specific immunosuppression.
Conflict of interest statement
Figures
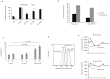
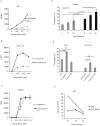
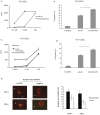
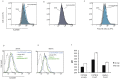

Similar articles
-
MHC Class II-Restricted Epitopes Containing an Oxidoreductase Activity Prompt CD4(+) T Cells with Apoptosis-Inducing Properties.Front Immunol. 2015 Sep 2;6:449. doi: 10.3389/fimmu.2015.00449. eCollection 2015. Front Immunol. 2015. PMID: 26388872 Free PMC article. Review.
-
The alloreactive and self-restricted CD4+ T cell response directed against a single MHC class II/peptide combination.J Immunol. 2000 Aug 1;165(3):1285-93. doi: 10.4049/jimmunol.165.3.1285. J Immunol. 2000. PMID: 10903728
-
CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner.J Immunol. 2003 Nov 1;171(9):4604-12. doi: 10.4049/jimmunol.171.9.4604. J Immunol. 2003. PMID: 14568934
-
IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells.Cell Immunol. 2009;257(1-2):69-79. doi: 10.1016/j.cellimm.2009.03.002. Epub 2009 Mar 31. Cell Immunol. 2009. PMID: 19338979 Free PMC article.
-
Cytotoxic T lymphocytes: the newly identified Fas (CD95)-mediated killing mechanism and a novel aspect of their biological functions.Adv Immunol. 1995;60:289-321. doi: 10.1016/s0065-2776(08)60588-x. Adv Immunol. 1995. PMID: 8607372 Review. No abstract available.
Cited by
-
Cytotoxic CD4 T Cells-Friend or Foe during Viral Infection?Front Immunol. 2017 Jan 23;8:19. doi: 10.3389/fimmu.2017.00019. eCollection 2017. Front Immunol. 2017. PMID: 28167943 Free PMC article. Review.
-
The Emerging Jamboree of Transformative Therapies for Autoimmune Diseases.Front Immunol. 2020 Mar 31;11:472. doi: 10.3389/fimmu.2020.00472. eCollection 2020. Front Immunol. 2020. PMID: 32296421 Free PMC article. Review.
-
MHC Class II-Restricted Epitopes Containing an Oxidoreductase Activity Prompt CD4(+) T Cells with Apoptosis-Inducing Properties.Front Immunol. 2015 Sep 2;6:449. doi: 10.3389/fimmu.2015.00449. eCollection 2015. Front Immunol. 2015. PMID: 26388872 Free PMC article. Review.
-
Antigen-specific therapeutic approaches for autoimmunity.Nat Biotechnol. 2019 Mar;37(3):238-251. doi: 10.1038/s41587-019-0015-4. Epub 2019 Feb 25. Nat Biotechnol. 2019. PMID: 30804535 Review.
-
Thioreductase-Containing Epitopes Inhibit the Development of Type 1 Diabetes in the NOD Mouse Model.Front Immunol. 2016 Mar 2;7:67. doi: 10.3389/fimmu.2016.00067. eCollection 2016. Front Immunol. 2016. PMID: 26973647 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

