DENV inhibits type I IFN production in infected cells by cleaving human STING
- PMID: 23055924
- PMCID: PMC3464218
- DOI: 10.1371/journal.ppat.1002934
DENV inhibits type I IFN production in infected cells by cleaving human STING
Abstract
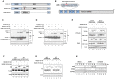
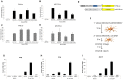
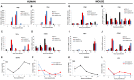
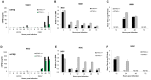
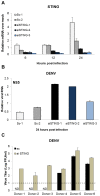
Dengue virus (DENV) is a pathogen with a high impact on human health. It replicates in a wide range of cells involved in the immune response. To efficiently infect humans, DENV must evade or inhibit fundamental elements of the innate immune system, namely the type I interferon response. DENV circumvents the host immune response by expressing proteins that antagonize the cellular innate immunity. We have recently documented the inhibition of type I IFN production by the proteolytic activity of DENV NS2B3 protease complex in human monocyte derived dendritic cells (MDDCs). In the present report we identify the human adaptor molecule STING as a target of the NS2B3 protease complex. We characterize the mechanism of inhibition of type I IFN production in primary human MDDCs by this viral factor. Using different human and mouse primary cells lacking STING, we show enhanced DENV replication. Conversely, mutated versions of STING that cannot be cleaved by the DENV NS2B3 protease induced higher levels of type I IFN after infection with DENV. Additionally, we show that DENV NS2B3 is not able to degrade the mouse version of STING, a phenomenon that severely restricts the replication of DENV in mouse cells, suggesting that STING plays a key role in the inhibition of DENV infection and spread in mice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Endoplasmic Reticulum Protein SCAP Inhibits Dengue Virus NS2B3 Protease by Suppressing Its K27-Linked Polyubiquitylation.J Virol. 2017 Apr 13;91(9):e02234-16. doi: 10.1128/JVI.02234-16. Print 2017 May 1. J Virol. 2017. PMID: 28228593 Free PMC article.
-
Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex.J Virol. 2010 Oct;84(19):9760-74. doi: 10.1128/JVI.01051-10. Epub 2010 Jul 21. J Virol. 2010. PMID: 20660196 Free PMC article.
-
Innate immunity evasion by Dengue virus.Viruses. 2012 Mar;4(3):397-413. doi: 10.3390/v4030397. Epub 2012 Mar 15. Viruses. 2012. PMID: 22590678 Free PMC article. Review.
-
Generation and Characterization of Human-Mouse STING Chimeras That Allow DENV Replication in Mouse Cells.mSphere. 2022 Jun 29;7(3):e0091421. doi: 10.1128/msphere.00914-21. Epub 2022 Apr 28. mSphere. 2022. PMID: 35477320 Free PMC article.
-
How Dengue Virus Circumvents Innate Immunity.Front Immunol. 2018 Dec 4;9:2860. doi: 10.3389/fimmu.2018.02860. eCollection 2018. Front Immunol. 2018. PMID: 30564245 Free PMC article. Review.
Cited by
-
Advances in crosstalk among innate immune pathways activated by mitochondrial DNA.Heliyon. 2024 Jan 3;10(1):e24029. doi: 10.1016/j.heliyon.2024.e24029. eCollection 2024 Jan 15. Heliyon. 2024. PMID: 38268572 Free PMC article. Review.
-
Cytosolic sensing of viruses.Immunity. 2013 May 23;38(5):855-69. doi: 10.1016/j.immuni.2013.05.007. Immunity. 2013. PMID: 23706667 Free PMC article. Review.
-
Camouflage and interception: how pathogens evade detection by intracellular nucleic acid sensors.Immunology. 2019 Mar;156(3):217-227. doi: 10.1111/imm.13030. Epub 2018 Dec 18. Immunology. 2019. PMID: 30499584 Free PMC article. Review.
-
Low-dimensional representation of genomic sequences.J Math Biol. 2019 Jul;79(1):1-29. doi: 10.1007/s00285-019-01348-1. Epub 2019 Mar 30. J Math Biol. 2019. PMID: 30929047
-
Avian IRF1 and IRF7 Play Overlapping and Distinct Roles in Regulating IFN-Dependent and -Independent Antiviral Responses to Duck Tembusu Virus Infection.Viruses. 2022 Jul 9;14(7):1506. doi: 10.3390/v14071506. Viruses. 2022. PMID: 35891486 Free PMC article.
References
-
- Ho LJ, Wang JJ, Shaio MF, Kao CL, Chang DM, et al. (2001) Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J Immunol 166: 1499–1506. - PubMed
-
- Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, et al. (2000) Human skin Langerhans cells are targets of dengue virus infection. Nat Med 6: 816–820. - PubMed
-
- Jessie K, Fong MY, Devi S, Lam SK, Wong KT (2004) Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis 189: 1411–1418. - PubMed
-
- Kou Z, Quinn M, Chen H, Rodrigo WW, Rose RC, et al. (2008) Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J Med Virol 80: 134–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

