Myeloid-derived suppressor cells regulate natural killer cell response to adenovirus-mediated gene transfer
- PMID: 23055553
- PMCID: PMC3503091
- DOI: 10.1128/JVI.01595-12
Myeloid-derived suppressor cells regulate natural killer cell response to adenovirus-mediated gene transfer
Abstract
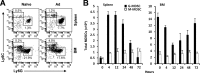
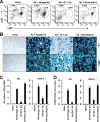
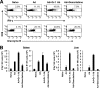
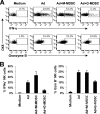
The attendant innate and adaptive immune responses to viral vectors have posed a significant hurdle for clinical application of viral vector-mediated gene therapy. Previous studies have shown that natural killer (NK) cells play a critical role in innate immune elimination of adenoviral vectors in the liver. However, it is not clear how the NK cell response to adenoviral vectors is regulated. In this study, we identified a role for granulocytic myeloid-derived suppressor cells (G-MDSCs) in this process. We show that in vivo administration of adenoviral vectors results in rapid accumulation of G-MDSCs early during adenoviral infection. In vivo depletion of both MDSC populations, but not monocytic MDSCs (M-MDSCs) alone, resulted in accelerated clearance of adenoviral vectors in the liver. This was accompanied by enhanced NK cell proliferation and activation, suggesting a role for MDSCs, probably G-MDSCs, in suppressing NK cell activation and function in vivo. We further demonstrate in vitro that G-MDSCs, but not M-MDSCs, are responsible for the suppression of NK cell activation. In addition, we show that adenoviral infection activated G-MDSCs to produce higher levels of reactive oxygen species (ROS) and that G-MDSC-mediated suppression of NK cells is mediated by ROS, specifically, H(2)O(2). This study demonstrates for the first time that the NK cell response to adenoviral vectors is negatively regulated by G-MDSCs and suggests that G-MDSC-based strategies could potentially improve the outcome of viral vector-mediated gene therapy.
Figures


Similar articles
-
NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells.J Immunol. 2012 Aug 15;189(4):1843-9. doi: 10.4049/jimmunol.1200584. Epub 2012 Jul 13. J Immunol. 2012. PMID: 22798671 Free PMC article.
-
A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo.Mol Ther. 2008 Jul;16(7):1300-7. doi: 10.1038/mt.2008.88. Epub 2008 Apr 29. Mol Ther. 2008. PMID: 18443600
-
NKG2D is required for NK cell activation and function in response to E1-deleted adenovirus.J Immunol. 2010 Dec 15;185(12):7480-6. doi: 10.4049/jimmunol.1002771. Epub 2010 Nov 12. J Immunol. 2010. PMID: 21076062 Free PMC article.
-
Myeloid-Derived Suppressor Cells in Trypanosoma cruzi Infection.Front Cell Infect Microbiol. 2021 Aug 27;11:737364. doi: 10.3389/fcimb.2021.737364. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34513737 Free PMC article. Review.
-
Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression.Front Immunol. 2019 Apr 18;10:771. doi: 10.3389/fimmu.2019.00771. eCollection 2019. Front Immunol. 2019. PMID: 31057536 Free PMC article. Review.
Cited by
-
Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome.Crit Care Med. 2017 Feb;45(2):253-262. doi: 10.1097/CCM.0000000000002074. Crit Care Med. 2017. PMID: 27632674 Free PMC article.
-
Ly6Chi monocytes regulate T cell responses in viral hepatitis.JCI Insight. 2016 Oct 20;1(17):e89880. doi: 10.1172/jci.insight.89880. JCI Insight. 2016. PMID: 27777980 Free PMC article.
-
Immunosenescence: the potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency.Cell Mol Life Sci. 2019 May;76(10):1901-1918. doi: 10.1007/s00018-019-03048-x. Epub 2019 Feb 20. Cell Mol Life Sci. 2019. PMID: 30788516 Free PMC article. Review.
-
Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms.Br J Cancer. 2014 Jan 7;110(1):83-93. doi: 10.1038/bjc.2013.695. Epub 2013 Nov 26. Br J Cancer. 2014. PMID: 24281006 Free PMC article.
-
Phenotype and Function of Myeloid-Derived Suppressor Cells Induced by Porphyromonas gingivalis Infection.Infect Immun. 2017 Jul 19;85(8):e00213-17. doi: 10.1128/IAI.00213-17. Print 2017 Aug. Infect Immun. 2017. PMID: 28533469 Free PMC article.
References
-
- Bowen JL, Olson JK. 2009. Innate immune CD11b+Gr-1+ cells, suppressor cells, affect the immune response during Theiler's virus-induced demyelinating disease. J. Immunol. 183:6971–6980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

