The leukemia-associated fusion protein MN1-TEL blocks TEL-specific recognition sequences
- PMID: 23049943
- PMCID: PMC3458806
- DOI: 10.1371/journal.pone.0046085
The leukemia-associated fusion protein MN1-TEL blocks TEL-specific recognition sequences
Abstract
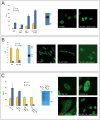
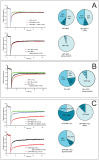
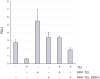
The leukemia-associated fusion protein MN1-TEL combines the transcription-activating domains of MN1 with the DNA-binding domain of the transcriptional repressor TEL. Quantitative photobleaching experiments revealed that ∼20% of GFP-tagged MN1 and TEL is transiently immobilised, likely due to indirect or direct DNA binding, since transcription inhibition abolished immobilisation. Interestingly, ∼50% of the MN1-TEL fusion protein was immobile with much longer binding times than unfused MN1 and TEL. MN1-TEL immobilisation was not observed when the TEL DNA-binding domain was disrupted, suggesting that MN1-TEL stably occupies TEL recognition sequences, preventing binding of factors required for proper transcription regulation, which may contribute to leukemogenesis.
Conflict of interest statement
Figures
Similar articles
-
Conditional MN1-TEL knock-in mice develop acute myeloid leukemia in conjunction with overexpression of HOXA9.Blood. 2005 Dec 15;106(13):4269-77. doi: 10.1182/blood-2005-04-1679. Epub 2005 Aug 16. Blood. 2005. PMID: 16105979 Free PMC article.
-
The MN1-TEL fusion protein, encoded by the translocation (12;22)(p13;q11) in myeloid leukemia, is a transcription factor with transforming activity.Mol Cell Biol. 2000 Dec;20(24):9281-93. doi: 10.1128/MCB.20.24.9281-9293.2000. Mol Cell Biol. 2000. PMID: 11094079 Free PMC article.
-
Overexpression of TEL-MN1 Fusion Enhances Resistance of HL-60 Cells to Idarubicin.Chemotherapy. 2018;63(6):308-314. doi: 10.1159/000495073. Epub 2019 Mar 6. Chemotherapy. 2018. PMID: 30840968
-
ETV6: a versatile player in leukemogenesis.Semin Cancer Biol. 2005 Jun;15(3):162-74. doi: 10.1016/j.semcancer.2005.01.008. Semin Cancer Biol. 2005. PMID: 15826831 Review.
-
Proteins of the ETS family with transcriptional repressor activity.Oncogene. 2000 Dec 18;19(55):6524-32. doi: 10.1038/sj.onc.1204045. Oncogene. 2000. PMID: 11175368 Review.
Cited by
-
Molecular mechanisms of ETS transcription factor-mediated tumorigenesis.Crit Rev Biochem Mol Biol. 2013 Nov-Dec;48(6):522-43. doi: 10.3109/10409238.2013.838202. Epub 2013 Sep 25. Crit Rev Biochem Mol Biol. 2013. PMID: 24066765 Free PMC article. Review.
References
-
- Buijs A, Sherr S, van Baal S, van Bezouw S, van der Plas D, et al. (1995) Translocation (12;22) (p13;q11) in myeloproliferative disorders results in fusion of the ETS-like TEL gene on 12p13 to the MN1 gene on 22q11 [published erratum appeared in Oncogene 1995 Aug 17;11(4): 809]. Oncogene 10: 1511–1519. - PubMed
-
- Chakrabarti SR, Nucifora G (1999) The leukemia-associated gene TEL encodes a transcription repressor which associates with SMRT and mSin3A. Biochem Biophys Res Commun 264: 871–877. - PubMed
-
- Janssen JW, Ridge SA, Papadopoulos P, Cotter F, Ludwig WD, et al. (1995) The fusion of TEL and ABL in human acute lymphoblastic leukaemia is a rare event. Br J Haematol 90: 222–224. - PubMed
-
- Bohlander SK (2005) ETV6: a versatile player in leukemogenesis. Semin Cancer Biol 15: 162–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

