The critical role of Astragalus polysaccharides for the improvement of PPARα [ correction of PPRAα]-mediated lipotoxicity in diabetic cardiomyopathy
- PMID: 23049681
- PMCID: PMC3462191
- DOI: 10.1371/journal.pone.0045541
The critical role of Astragalus polysaccharides for the improvement of PPARα [ correction of PPRAα]-mediated lipotoxicity in diabetic cardiomyopathy
Abstract
Background: Obesity-related diabetes mellitus leads to increased myocardial uptake and oxidation of fatty acids, resulting in a form of cardiac dysfunction referred to as lipotoxic cardiomyopathy. We have shown previously that Astragalus polysaccharides (APS) administration was sufficient to improve the systemic metabolic disorder and cardiac dysfunction in diabetic models.
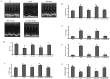
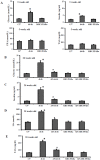
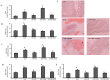
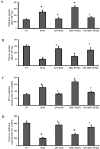
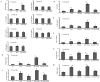
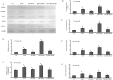
Methodology/principal findings: To investigate the precise role of APS therapy in the pathogenesis of myocardial lipotoxity in diabetes, db/db diabetic mice and myosin heavy chain (MHC)- peroxisome proliferator-activated receptor (PPAR) α mice were characterized and administrated with or without APS with C57 wide- type mice as normal control. APS treatment strikingly improved the myocyte triacylglyceride accumulation and cardiac dysfunction in both db/db mice and MHC-PPARα mice, with the normalization of energy metabolic derangements in both db/db diabetic hearts and MHC-PPARα hearts. Consistently, the activation of PPARα target genes involved in myocardial fatty acid uptake and oxidation in both db/db diabetic hearts and MHC-PPARα hearts was reciprocally repressed by APS administration, while PPARα-mediated suppression of genes involved in glucose utilization of both diabetic hearts and MHC-PPARα hearts was reversed by treatment with APS.
Conclusions: We conclude that APS therapy could prevent the development of diabetic cardiomyopathy through a mechanism mainly dependent on the cardiac PPARα-mediated regulatory pathways.
Conflict of interest statement
Figures
Similar articles
-
Astragalus polysaccharides repress myocardial lipotoxicity in a PPARalpha-dependent manner in vitro and in vivo in mice.J Diabetes Complications. 2015 Mar;29(2):164-75. doi: 10.1016/j.jdiacomp.2014.11.007. Epub 2014 Nov 25. J Diabetes Complications. 2015. PMID: 25499591
-
Therapy with Astragalus polysaccharides rescues lipotoxic cardiomyopathy in MHC-PPARα mice.Mol Biol Rep. 2013 Mar;40(3):2449-59. doi: 10.1007/s11033-012-2325-1. Epub 2012 Nov 30. Mol Biol Rep. 2013. PMID: 23196711
-
CD36 deficiency rescues lipotoxic cardiomyopathy.Circ Res. 2007 Apr 27;100(8):1208-17. doi: 10.1161/01.RES.0000264104.25265.b6. Epub 2007 Mar 15. Circ Res. 2007. PMID: 17363697
-
The role of the peroxisome proliferator-activated receptor alpha pathway in pathological remodeling of the diabetic heart.Curr Opin Clin Nutr Metab Care. 2004 Jul;7(4):391-6. doi: 10.1097/01.mco.0000134371.70815.32. Curr Opin Clin Nutr Metab Care. 2004. PMID: 15192440 Review.
-
Fatty acid metabolism is enhanced in type 2 diabetic hearts.Biochim Biophys Acta. 2005 May 15;1734(2):112-26. doi: 10.1016/j.bbalip.2005.03.005. Epub 2005 Apr 9. Biochim Biophys Acta. 2005. PMID: 15904868 Review.
Cited by
-
Metabolic dysfunction in diabetic cardiomyopathy.Heart Fail Rev. 2014 Jan;19(1):35-48. doi: 10.1007/s10741-013-9377-8. Heart Fail Rev. 2014. PMID: 23443849 Free PMC article. Review.
-
Emodin protects against diabetic cardiomyopathy by regulating the AKT/GSK-3β signaling pathway in the rat model.Molecules. 2014 Sep 17;19(9):14782-93. doi: 10.3390/molecules190914782. Molecules. 2014. PMID: 25232702 Free PMC article.
-
Beneficial Effects of Echinacoside on Diabetic Cardiomyopathy in Diabetic Db/Db Mice.Drug Des Devel Ther. 2020 Dec 18;14:5575-5587. doi: 10.2147/DDDT.S276972. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33376302 Free PMC article.
-
Effect of Gegen Qinlian Decoction on Cardiac Gene Expression in Diabetic Mice.Int J Genomics. 2017;2017:7421761. doi: 10.1155/2017/7421761. Epub 2017 Dec 12. Int J Genomics. 2017. PMID: 29379793 Free PMC article.
-
High-throughput analysis and characterization of Astragalus membranaceus transcriptome using 454 GS FLX.PLoS One. 2014 May 14;9(5):e95831. doi: 10.1371/journal.pone.0095831. eCollection 2014. PLoS One. 2014. PMID: 24828103 Free PMC article.
References
-
- Witteles RM, Fowler MB (2008) Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol 51(2): 93–102. - PubMed
-
- Carley AN, Severson DL (2005) Fatty acid metabolsim is enhanced in type 2 diabetic hearts. Biochim Biophys Acta 1734: 112–126. - PubMed
-
- Lopaschuk GD (2002) Metabolic abnormalities in the diabetic heart. Heart Fail Rev 7: 149–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

