Targeting JNK-interacting-protein-1 (JIP1) sensitises osteosarcoma to doxorubicin
- PMID: 23045411
- PMCID: PMC3717953
- DOI: 10.18632/oncotarget.600
Targeting JNK-interacting-protein-1 (JIP1) sensitises osteosarcoma to doxorubicin
Abstract
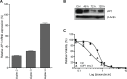
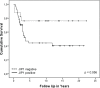
Osteosarcoma (OS) is the most common primary malignant bone tumour in children and adolescents. Despite aggressive therapy, survival outcomes remain unsatisfactory, especially for patients with metastatic disease or patients with a poor chemotherapy response. Chemoresistance contributes to treatment failure. To increase the efficacy of conventional chemotherapy, essential survival pathways should be targeted concomitantly. Here, we performed a loss-of-function siRNA screen of the human kinome in SaOS-2 cells to identify critical survival kinases after doxorubicin treatment. Gene silencing of JNK-interacting-protein-1 (JIP1) elicited the most potent sensitisation to doxorubicin. This candidate was further explored as potential target for chemosensitisation in OS. A panel of OS cell lines and human primary osteoblasts was examined for sensitisation to doxorubicin using small molecule JIP1-inhibitor BI-78D3. JIP1 expression and JIP1-inhibitor effects on JNK-signalling were investigated by Western blot analysis. JIP1 expression in human OS tumours was assessed by immunohistochemistry on tissue micro arrays. BI-78D3 blocked JNK-signalling and sensitised three out of four tested OS cell lines, but not healthy osteoblasts, to treatment with doxorubicin. Combination treatment increased the induction of apoptosis. JIP1 was found to be expressed in two-thirds of human primary OS tissue samples. Patients with JIP1 positive tumours showed a trend to inferior overall survival. Collectively, JIP1 appears a clinically relevant novel target in OS to enhance the efficacy of doxorubicin treatment by means of RNA interference or pharmacological inhibition.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

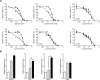

Similar articles
-
Codelivery of doxorubicin and JIP1 siRNA with novel EphA2-targeted PEGylated cationic nanoliposomes to overcome osteosarcoma multidrug resistance.Int J Nanomedicine. 2018 Jul 3;13:3853-3866. doi: 10.2147/IJN.S150017. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30013340 Free PMC article.
-
Long non-coding RNA CTA sensitizes osteosarcoma cells to doxorubicin through inhibition of autophagy.Oncotarget. 2017 May 9;8(19):31465-31477. doi: 10.18632/oncotarget.16356. Oncotarget. 2017. PMID: 28415557 Free PMC article.
-
TGF-β1-induced miR-202 mediates drug resistance by inhibiting apoptosis in human osteosarcoma.J Cancer Res Clin Oncol. 2016 Jan;142(1):239-46. doi: 10.1007/s00432-015-2028-9. Epub 2015 Aug 15. J Cancer Res Clin Oncol. 2016. PMID: 26276504
-
Targeting ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the CRISPR-Cas9 system to reverse drug resistance.Oncotarget. 2016 Dec 13;7(50):83502-83513. doi: 10.18632/oncotarget.13148. Oncotarget. 2016. PMID: 27835872 Free PMC article. Review.
-
Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies.Int J Mol Sci. 2020 Sep 19;21(18):6885. doi: 10.3390/ijms21186885. Int J Mol Sci. 2020. PMID: 32961800 Free PMC article. Review.
Cited by
-
Targeting protein kinases to reverse multidrug resistance in sarcoma.Cancer Treat Rev. 2016 Feb;43:8-18. doi: 10.1016/j.ctrv.2015.11.011. Epub 2015 Dec 8. Cancer Treat Rev. 2016. PMID: 26827688 Free PMC article. Review.
-
Exploration of gastric neuroendocrine carcinoma (GNEC) specific signaling pathways involved in chemoresistance via transcriptome and in vitro analysis.Comput Struct Biotechnol J. 2020 Sep 20;18:2610-2620. doi: 10.1016/j.csbj.2020.09.016. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33033581 Free PMC article.
-
A-770041 reverses paclitaxel and doxorubicin resistance in osteosarcoma cells.BMC Cancer. 2014 Sep 19;14:681. doi: 10.1186/1471-2407-14-681. BMC Cancer. 2014. PMID: 25236161 Free PMC article.
-
Selective inhibitors for JNK signalling: a potential targeted therapy in cancer.J Enzyme Inhib Med Chem. 2020 Dec;35(1):574-583. doi: 10.1080/14756366.2020.1720013. J Enzyme Inhib Med Chem. 2020. PMID: 31994958 Free PMC article. Review.
-
Recent advances in delivery of drug-nucleic acid combinations for cancer treatment.J Control Release. 2013 Dec 10;172(2):589-600. doi: 10.1016/j.jconrel.2013.04.010. Epub 2013 Apr 25. J Control Release. 2013. PMID: 23624358 Free PMC article. Review.
References
-
- Bacci G, Ferrari S, Bertoni F, Picci P, Bacchini P, Longhi A, Donati D, Forni C, Campanacci L, Campanacci M. Histologic response of high-grade nonmetastatic osteosarcoma of the extremity to chemotherapy. Clin. Orthop. Relat Res. 2001:186–196. - PubMed
-
- Bacci G, Rocca M, Salone M, Balladelli A, Ferrari S, Palmerini E, Forni C, Briccoli A. High grade osteosarcoma of the extremities with lung metastases at presentation: treatment with neoadjuvant chemotherapy and simultaneous resection of primary and metastatic lesions. J. Surg. Oncol. 2008;98:415–420. - PubMed
-
- Bielack S. S, Kempf-Bielack B, Delling G, Exner G. U, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20:776–790. - PubMed
-
- Bielack S. S, Carrle D, Hardes J, Schuck A, Paulussen M. Bone tumors in adolescents and young adults. Curr. Treat. Options. Oncol. 2008;9:67–80. - PubMed
-
- Scotlandi K, Picci P, Kovar H. Targeted therapies in bone sarcomas. Curr. Cancer Drug Targets. 2009;9:843–853. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

