Quantitative LC-MS/MS analysis of arachidonoyl amino acids in mouse brain with treatment of FAAH inhibitor
- PMID: 23044255
- PMCID: PMC3760509
- DOI: 10.1016/j.ab.2012.09.031
Quantitative LC-MS/MS analysis of arachidonoyl amino acids in mouse brain with treatment of FAAH inhibitor
Abstract
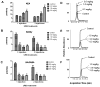
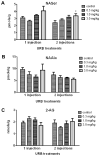
An additional class of endogenous lipid amides, N-arachidonoyl amino acids (Ara-AAs), is growing in significance in the field of endocannabinoids. The development, validation, and application of a sensitive and selective method to simultaneously monitor and quantify the level of Ara-AAs along with anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) in mouse brain has been established. The linearity of the method over the concentration ranges of 0.2-120 pg/μl for the standards of N-arachidonoyl amino acids, N-arachidonoyl alanine (NAAla), serine (NASer), γ-aminobutyric acid (NAGABA), and glycine (NAGly); 0.7-90 pg/μl for AEA-d(0)/d(8); and 7.5-950 pg/μl for 2-AG was determined with R(2) values of 0.99. Also the effects of the FAAH inhibitor URB 597 on the endogenous levels of these analytes were investigated. AEA and NASer brain levels exhibit a dose-dependent increase after systemic administration of URB 597, whereas NAGly and NAGABA were significantly decreased after treatment. NAAla and 2-AG were not altered after URB 597 treatment. The potential benefit of establishing this assay extends beyond the quantification of the Ara-AAs along with AEA and 2-AG in mouse brain, to reveal a variety of pharmacological effects and physiological roles of these analytes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


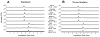
Similar articles
-
The endocannabinoid anandamide is a precursor for the signaling lipid N-arachidonoyl glycine by two distinct pathways.BMC Biochem. 2009 May 21;10:14. doi: 10.1186/1471-2091-10-14. BMC Biochem. 2009. PMID: 19460156 Free PMC article.
-
Induction of endocannabinoid levels in juvenile rat brain following developmental chlorpyrifos exposure.Toxicol Sci. 2013 Sep;135(1):193-201. doi: 10.1093/toxsci/kft126. Epub 2013 Jun 12. Toxicol Sci. 2013. PMID: 23761300 Free PMC article.
-
A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids.Rapid Commun Mass Spectrom. 2015 Oct 30;29(20):1889-97. doi: 10.1002/rcm.7277. Rapid Commun Mass Spectrom. 2015. PMID: 26411510
-
Fatty acid amide hydrolase, an enzyme with many bioactive substrates. Possible therapeutic implications.Curr Pharm Des. 2002;8(7):533-47. doi: 10.2174/1381612023395655. Curr Pharm Des. 2002. PMID: 11945157 Review.
-
Fatty acid amide hydrolase: biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2-arachidonoylglycerol, palmitoylethanolamide, and oleamide.Biochem Pharmacol. 2001 Sep 1;62(5):517-26. doi: 10.1016/s0006-2952(01)00712-2. Biochem Pharmacol. 2001. PMID: 11585048 Review.
Cited by
-
Activation of the endocannabinoid system mediates cardiac hypertrophy induced by rosiglitazone.Acta Pharmacol Sin. 2022 Sep;43(9):2302-2312. doi: 10.1038/s41401-022-00858-x. Epub 2022 Feb 21. Acta Pharmacol Sin. 2022. PMID: 35190698 Free PMC article.
-
Evidence for a GPR18 Role in Chemotaxis, Proliferation, and the Course of Wound Closure in the Cornea.Cornea. 2019 Jul;38(7):905-913. doi: 10.1097/ICO.0000000000001934. Cornea. 2019. PMID: 30969262 Free PMC article.
-
Environmental Toxin Acrolein Alters Levels of Endogenous Lipids, Including TRP Agonists: A Potential Mechanism for Headache Driven by TRPA1 Activation.Neurobiol Pain. 2017 Jan-Jul;1:28-36. doi: 10.1016/j.ynpai.2017.03.001. Epub 2017 May 17. Neurobiol Pain. 2017. PMID: 29430557 Free PMC article.
-
Investigation of vitamin B₆ inadequacy, induced by exposure to the anti-B₆ factor 1-amino D-proline, on plasma lipophilic metabolites of rats: a metabolomics approach.Eur J Nutr. 2016 Apr;55(3):1213-23. doi: 10.1007/s00394-015-0934-x. Epub 2015 May 26. Eur J Nutr. 2016. PMID: 26009005
-
Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain.Molecules. 2021 Apr 6;26(7):2086. doi: 10.3390/molecules26072086. Molecules. 2021. PMID: 33917316 Free PMC article.
References
-
- Patricelli MP, Cravatt BF. Proteins regulating the biosynthesis and inactivation of neuromodulatory fatty acid amides. Vitam Horm. 2001;62:95–131. - PubMed
-
- Yates ML, Barker EL. Inactivation and biotransformation of the endogenous cannabinoids anandamide and 2-arachidonoylglycerol. Mol Pharmacol. 2009;76:11–17. - PubMed
-
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. - PubMed
-
- Fontana A, Di Marzo V, Cadas H, Piomelli D. Analysis of anandamide, an endogenous cannabinoid substance, and of other natural N-acylethanolamines. Prostaglandins Leukot Essent Fatty Acids. 1995;53:301–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

