Radiation combined with thermal injury induces immature myeloid cells
- PMID: 23042190
- PMCID: PMC3607646
- DOI: 10.1097/SHK.0b013e31826c5b19
Radiation combined with thermal injury induces immature myeloid cells
Abstract
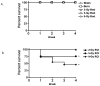
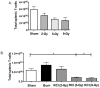
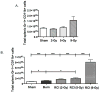
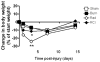
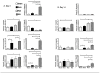
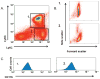
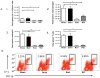
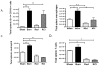
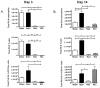
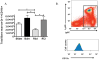
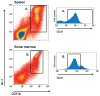
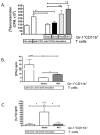
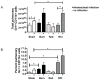
The continued development of nuclear weapons and the potential for thermonuclear injury necessitates the further understanding of the immune consequences after radiation combined with injury (RCI). We hypothesized that sublethal ionization radiation exposure combined with a full-thickness thermal injury would result in the production of immature myeloid cells. Mice underwent either a full-thickness contact burn of 20% total body surface area or sham procedure followed by a single whole-body dose of 5-Gy radiation. Serum, spleen, and peripheral lymph nodes were harvested at 3 and 14 days after injury. Flow cytometry was performed to identify and characterize adaptive and innate cell compartments. Elevated proinflammatory and anti-inflammatory serum cytokines and profound leukopenia were observed after RCI. A population of cells with dual expression of the cell surface markers Gr-1 and CD11b were identified in all experimental groups, but were significantly elevated after burn alone and RCI at 14 days after injury. In contrast to the T-cell-suppressive nature of myeloid-derived suppressor cells found after trauma and sepsis, myeloid cells after RCI augmented T-cell proliferation and were associated with a weak but significant increase in interferon γ and a decrease in interleukin 10. This is consistent with previous work in burn injury indicating that a myeloid-derived suppressor cell-like population increases innate immunity. Radiation combined injury results in the increase in distinct populations of Gr-1CD11b cells within the secondary lymphoid organs, and we propose these immature inflammatory myeloid cells provide innate immunity to the severely injured and immunocompromised host.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures

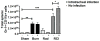

Similar articles
-
Immune system phenotyping of radiation and radiation combined injury in outbred mice.Radiat Res. 2013 Jan;179(1):101-12. doi: 10.1667/RR3120.1. Epub 2012 Dec 5. Radiat Res. 2013. PMID: 23216446 Free PMC article.
-
Changes in lymphocyte number and phenotype in seven lymphoid compartments after thermal injury.Ann Surg. 1989 Jul;210(1):78-89. doi: 10.1097/00000658-198907000-00012. Ann Surg. 1989. PMID: 2742415 Free PMC article.
-
Immature myeloid Gr-1+ CD11b+ cells from lipopolysaccharide-immunosuppressed mice acquire inhibitory activity in the bone marrow and migrate to lymph nodes to exert their suppressive function.Clin Sci (Lond). 2016 Feb;130(4):259-71. doi: 10.1042/CS20150653. Epub 2015 Nov 18. Clin Sci (Lond). 2016. PMID: 26582821
-
Inhibition of PPARγ in myeloid-lineage cells induces systemic inflammation, immunosuppression, and tumorigenesis.Blood. 2012 Jan 5;119(1):115-26. doi: 10.1182/blood-2011-06-363093. Epub 2011 Nov 3. Blood. 2012. PMID: 22053106 Free PMC article.
-
Radiation combined injury: overview of NIAID research.Health Phys. 2010 Jun;98(6):863-7. doi: 10.1097/HP.0b013e3181a6ee32. Health Phys. 2010. PMID: 20445395 Free PMC article. Review.
Cited by
-
Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12- neutrophil polarization.PLoS One. 2014 Jan 15;9(1):e85623. doi: 10.1371/journal.pone.0085623. eCollection 2014. PLoS One. 2014. PMID: 24454904 Free PMC article.
-
One-hit wonder: Late after burn injury, granulocytes can clear one bacterial infection but cannot control a subsequent infection.Burns. 2019 May;45(3):627-640. doi: 10.1016/j.burns.2018.08.019. Epub 2019 Mar 2. Burns. 2019. PMID: 30833100 Free PMC article.
-
Innate Immune Cell Recovery Is Positively Regulated by NLRP12 during Emergency Hematopoiesis.J Immunol. 2017 Mar 15;198(6):2426-2433. doi: 10.4049/jimmunol.1601048. Epub 2017 Feb 3. J Immunol. 2017. PMID: 28159904 Free PMC article.
-
Will mesenchymal stem cells be future directions for treating radiation-induced skin injury?Stem Cell Res Ther. 2021 Mar 12;12(1):179. doi: 10.1186/s13287-021-02261-5. Stem Cell Res Ther. 2021. PMID: 33712078 Free PMC article. Review.
-
Characterization of the Basal and mTOR-Dependent Acute Pulmonary and Systemic Immune Response in a Murine Model of Combined Burn and Inhalation Injury.Int J Mol Sci. 2022 Aug 7;23(15):8779. doi: 10.3390/ijms23158779. Int J Mol Sci. 2022. PMID: 35955914 Free PMC article.
References
-
- Buchanan IB, Maile R, Frelinger JA, Fair JH, Meyer AA, Cairns BA. The effect of burn injury on CD8+ and CD4+ T cells in an irradiation model of homeostatic proliferation. 2006;61:1062–1068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

