Detection of eastern equine encephalomyelitis virus RNA in North American snakes
- PMID: 23033405
- PMCID: PMC3516089
- DOI: 10.4269/ajtmh.2012.12-0257
Detection of eastern equine encephalomyelitis virus RNA in North American snakes
Abstract
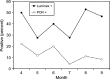
The role of non-avian vertebrates in the ecology of eastern equine encephalomyelitis virus (EEEV) is unresolved, but mounting evidence supports a potential role for snakes in the EEEV transmission cycle, especially as over-wintering hosts. To determine rates of exposure and infection, we examined serum samples from wild snakes at a focus of EEEV in Alabama for viral RNA using quantitative reverse transcription polymerase chain reaction. Two species of vipers, the copperhead (Agkistrodon contortrix) and the cottonmouth (Agkistrodon piscivorus), were found to be positive for EEEV RNA using this assay. Prevalence of EEEV RNA was more frequent in seropositive snakes than seronegative snakes. Positivity for the quantitative reverse transcription polymerase chain reaction in cottonmouths peaked in April and September. Body size and sex ratios were not significantly different between infected and uninfected snakes. These results support the hypothesis that snakes are involved in the ecology of EEEV in North America, possibly as over-wintering hosts for the virus.
Figures
Similar articles
-
Serosurveillance of eastern equine encephalitis virus in amphibians and reptiles from Alabama, USA.Am J Trop Med Hyg. 2012 Mar;86(3):540-4. doi: 10.4269/ajtmh.2012.11-0283. Am J Trop Med Hyg. 2012. PMID: 22403333 Free PMC article.
-
Competency of reptiles and amphibians for eastern equine encephalitis virus.Am J Trop Med Hyg. 2011 Sep;85(3):421-5. doi: 10.4269/ajtmh.2011.11-0006. Am J Trop Med Hyg. 2011. PMID: 21896798 Free PMC article.
-
Evolutionary patterns of eastern equine encephalitis virus in North versus South America suggest ecological differences and taxonomic revision.J Virol. 2010 Jan;84(2):1014-25. doi: 10.1128/JVI.01586-09. Epub 2009 Nov 4. J Virol. 2010. PMID: 19889755 Free PMC article.
-
Virology, ecology, epidemiology, pathology, and treatment of eastern equine encephalitis.J Neurol Sci. 2024 Feb 15;457:122886. doi: 10.1016/j.jns.2024.122886. Epub 2024 Jan 13. J Neurol Sci. 2024. PMID: 38278094 Review.
-
Eastern Equine Encephalitis Virus: A Case Report and Brief Literature Review of Current Therapeutic and Preventative Strategies.Vector Borne Zoonotic Dis. 2024 Feb;24(2):118-121. doi: 10.1089/vbz.2023.0011. Epub 2023 Oct 23. Vector Borne Zoonotic Dis. 2024. PMID: 37870590 Review.
Cited by
-
Host associations of mosquitoes at eastern equine encephalitis virus foci in Connecticut, USA.Parasit Vectors. 2016 Aug 30;9(1):474. doi: 10.1186/s13071-016-1765-1. Parasit Vectors. 2016. PMID: 27577939 Free PMC article.
-
Replication of Rift Valley Fever Virus in Amphibian and Reptile-Derived Cell Lines.Pathogens. 2021 May 31;10(6):681. doi: 10.3390/pathogens10060681. Pathogens. 2021. PMID: 34072763 Free PMC article.
-
A risk index model for predicting eastern equine encephalitis virus transmission to horses in Florida.Appl Geogr. 2014 Mar 1;48:79-86. doi: 10.1016/j.apgeog.2014.01.012. Appl Geogr. 2014. PMID: 24764607 Free PMC article.
-
Competency of Amphibians and Reptiles and Their Potential Role as Reservoir Hosts for Rift Valley Fever Virus.Viruses. 2020 Oct 23;12(11):1206. doi: 10.3390/v12111206. Viruses. 2020. PMID: 33114178 Free PMC article.
-
Complex Epidemiological Dynamics of Eastern Equine Encephalitis Virus in Florida.Am J Trop Med Hyg. 2019 May;100(5):1266-1274. doi: 10.4269/ajtmh.18-0783. Am J Trop Med Hyg. 2019. PMID: 30860014 Free PMC article.
References
-
- Bigler WJ, Lassing EB, Buff EE, Prather EC, Beck EC, Hoff GL. Endemic eastern equine encephalomyelitis in Florida: a twenty-year analysis, 1955–1974. Am J Trop Med Hyg. 1976;25:884–890. - PubMed
-
- Armstrong PM, Andreadis TG, Anderson JF, Stull JW, Mores CN. Tracking eastern equine encephalitis virus perpetuation in the northeastern United States by phylogenetic analysis. Am J Trop Med Hyg. 2008;79:291–296. - PubMed
-
- Weaver SC, Scott TW, Rico-Hesse R. Molecular evolution of eastern equine encephalomyelitis virus in North America. Virology. 1991;182:774–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

