Induction of the staphylococcal proteolytic cascade by antimicrobial fatty acids in community acquired methicillin resistant Staphylococcus aureus
- PMID: 23029337
- PMCID: PMC3454333
- DOI: 10.1371/journal.pone.0045952
Induction of the staphylococcal proteolytic cascade by antimicrobial fatty acids in community acquired methicillin resistant Staphylococcus aureus
Abstract
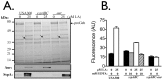
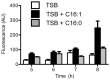
Community acquired methicillin resistant Staphylococcus aureus (CA-MRSA), and the USA300 strain of CA-MRSA in particular, are known for their rapid community transmission, and propensity to cause aggressive skin and soft tissue infections. To assess factors that contribute to these hallmark traits of CA-MRSA, we evaluated how growth of USA300 and production of secreted virulence factors was influenced on exposure to physiologic levels of unsaturated free fatty acids that would be encountered on the skin or anterior nares, which represent the first sites of contact with healthy human hosts. There was a sharp threshold between sub-inhibitory and inhibitory concentrations, such that 100 µM sapienic acid (C16∶1) and linoleic acid (C18∶1) were sufficient to prevent growth after 24 h incubation, while 25 µM allowed unrestricted growth, and 50 µM caused an approximate 10-12 h lag, followed by unimpeded exponential growth. Conversely, saturated palmitic or stearic acids did not affect growth at 100 µM. Although growth was not affected by 25 µM sapienic or linoleic acid, these and other unsaturated C16 and C18 fatty acids, but not their saturated counterparts, promoted robust production of secreted proteases comprising the Staphylococcal proteolytic cascade. This trait was also manifested to varying degrees in other CA-MRSA, and in genetically diverse methicillin susceptible S. aureus strains. Therefore, induction of the Staphylococcal proteolytic cascade by unsaturated fatty acids is another feature that should now be evaluated as a potential contributing factor in the aggressive nature of skin and soft tissue infections caused by USA300, and as a general virulence mechanism of S. aureus.
Conflict of interest statement
Figures



Similar articles
-
[Investigation of SCCmec types and Panton-Valentine leukocidin in community-acquired and nosocomial Staphylococcus aureus strains: comparing skin and soft tissue infections to the other infections].Mikrobiyol Bul. 2012 Jul;46(3):341-51. Mikrobiyol Bul. 2012. PMID: 22951646 Turkish.
-
Molecular characteristics of community-acquired methicillin-resistant Staphylococcus aureus strains isolated from outpatients with skin and soft tissue infections in Wuhan, China.Pathog Dis. 2016 Jun;74(4):ftw026. doi: 10.1093/femspd/ftw026. Epub 2016 Apr 7. Pathog Dis. 2016. PMID: 27060098
-
Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus.Annu Rev Microbiol. 2010;64:143-62. doi: 10.1146/annurev.micro.112408.134309. Annu Rev Microbiol. 2010. PMID: 20825344 Review.
-
Inhibition of Staphylococcus aureus Biofilm Formation and Virulence Factor Production by Petroselinic Acid and Other Unsaturated C18 Fatty Acids.Microbiol Spectr. 2022 Jun 29;10(3):e0133022. doi: 10.1128/spectrum.01330-22. Epub 2022 Jun 1. Microbiol Spectr. 2022. PMID: 35647620 Free PMC article.
-
Cutaneous community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus.Dermatol Ther. 2011 Mar-Apr;24(2):263-72. doi: 10.1111/j.1529-8019.2011.01402.x. Dermatol Ther. 2011. PMID: 21410616 Review.
Cited by
-
Prevalence and Characteristics of Staphylococcus aureus Isolated From Retail Raw Milk in Northern Xinjiang, China.Front Microbiol. 2021 Aug 9;12:705947. doi: 10.3389/fmicb.2021.705947. eCollection 2021. Front Microbiol. 2021. PMID: 34434176 Free PMC article.
-
Repeated Emergence of Variant TetR Family Regulator, FarR, and Increased Resistance to Antimicrobial Unsaturated Fatty Acid among Clonal Complex 5 Methicillin-Resistant Staphylococcus aureus.Antimicrob Agents Chemother. 2023 Mar 16;67(3):e0074922. doi: 10.1128/aac.00749-22. Epub 2023 Feb 6. Antimicrob Agents Chemother. 2023. PMID: 36744906 Free PMC article.
-
Role of lipase from community-associated methicillin-resistant Staphylococcus aureus strain USA300 in hydrolyzing triglycerides into growth-inhibitory free fatty acids.J Bacteriol. 2014 Dec;196(23):4044-56. doi: 10.1128/JB.02044-14. Epub 2014 Sep 15. J Bacteriol. 2014. PMID: 25225262 Free PMC article.
-
Superantigens Modulate Bacterial Density during Staphylococcus aureus Nasal Colonization.Toxins (Basel). 2015 May 22;7(5):1821-36. doi: 10.3390/toxins7051821. Toxins (Basel). 2015. PMID: 26008236 Free PMC article.
-
Novel Functions and Signaling Specificity for the GraS Sensor Kinase of Staphylococcus aureus in Response to Acidic pH.J Bacteriol. 2020 Oct 22;202(22):e00219-20. doi: 10.1128/JB.00219-20. Print 2020 Oct 22. J Bacteriol. 2020. PMID: 32868405 Free PMC article.
References
-
- Peacock SJ, de Silva I, Lowy FD (2001) What determines nasal carriage of Staphylococcus aureus? Trends Microbiol 9: 605–610. - PubMed
-
- Archer GL (1998) Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis 26: 1179–1181. - PubMed
-
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520–532. - PubMed
-
- Mertz D, Frei R, Jaussi B, Tietz A, Stebler C, et al. (2007) Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus . Clin Infect Dis 45: 475–477. - PubMed
-
- Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, et al. (2007) The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe 1: 199–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

