OSBP-related proteins (ORPs) in human adipose depots and cultured adipocytes: evidence for impacts on the adipocyte phenotype
- PMID: 23028956
- PMCID: PMC3448648
- DOI: 10.1371/journal.pone.0045352
OSBP-related proteins (ORPs) in human adipose depots and cultured adipocytes: evidence for impacts on the adipocyte phenotype
Abstract
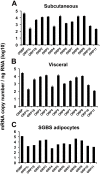
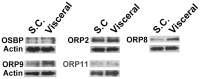
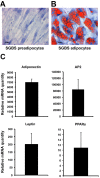
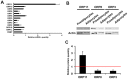
Oxysterol-binding protein (OSBP) homologues, ORPs, are implicated in lipid homeostatic control, vesicle transport, and cell signaling. We analyzed here the quantity of ORP mRNAs in human subcutaneous (s.c.) and visceral adipose depots, as well as in the Simpson-Golabi-Behmel syndrome (SGBS) adipocyte cell model. All of the ORP mRNAs were present in the s.c and visceral adipose tissues, and the two depots shared an almost identical ORP mRNA expression pattern. SGBS adipocytes displayed a similar pattern, suggesting that the adipose tissue ORP expression pattern mainly derives from adipocytes. During SGBS cell adipogenic differentiation, ORP2, ORP3, ORP4, ORP7, and ORP8 mRNAs were down-regulated, while ORP11 was induced. To assess the impacts of ORPs on adipocyte differentiation, ORP3 and ORP8, proteins down-regulated during adipogenesis, were overexpressed in differentiating SGBS adipocytes, while ORP11, a protein induced during adipogenesis, was silenced. ORP8 overexpression resulted in reduced expression of the aP2 mRNA, while down-regulation of adiponectin and aP2 was observed in ORP11 silenced cells. Furthermore, ORP8 overexpression or silencing of ORP11 markedly decreased cellular triglyceride storage. These data identify the patterns of ORP expression in human adipose depots and SGBS adipocytes, and provide the first evidence for a functional impact of ORPs on the adipocyte phenotype.
Conflict of interest statement
Figures
Similar articles
-
Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism.Prog Lipid Res. 2013 Oct;52(4):529-38. doi: 10.1016/j.plipres.2013.06.004. Epub 2013 Jul 2. Prog Lipid Res. 2013. PMID: 23830809 Review.
-
The mammalian oxysterol-binding protein-related proteins (ORPs) bind 25-hydroxycholesterol in an evolutionarily conserved pocket.Biochem J. 2007 Aug 1;405(3):473-80. doi: 10.1042/BJ20070176. Biochem J. 2007. PMID: 17428193 Free PMC article.
-
Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2.J Biol Chem. 2005 Apr 15;280(15):14656-62. doi: 10.1074/jbc.M412585200. Epub 2005 Feb 14. J Biol Chem. 2005. PMID: 15710620
-
Oxysterol-binding proteins: functions in cell regulation beyond lipid metabolism.Biochem Pharmacol. 2013 Jul 1;86(1):89-95. doi: 10.1016/j.bcp.2013.02.016. Epub 2013 Feb 18. Biochem Pharmacol. 2013. PMID: 23428468 Review.
-
Adipocyte-specific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance.Mol Metab. 2016 Sep 28;5(12):1149-1161. doi: 10.1016/j.molmet.2016.09.009. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900258 Free PMC article.
Cited by
-
ORP8 acts as a lipophagy receptor to mediate lipid droplet turnover.Protein Cell. 2023 Sep 14;14(9):653-667. doi: 10.1093/procel/pwac063. Protein Cell. 2023. PMID: 37707322 Free PMC article.
-
Bridging the molecular and biological functions of the oxysterol-binding protein family.Cell Mol Life Sci. 2018 Sep;75(17):3079-3098. doi: 10.1007/s00018-018-2795-y. Epub 2018 Mar 13. Cell Mol Life Sci. 2018. PMID: 29536114 Free PMC article. Review.
-
Severe neurodegenerative disease in brothers with homozygous mutation in POLR1A.Eur J Hum Genet. 2017 Feb;25(3):315-323. doi: 10.1038/ejhg.2016.183. Epub 2017 Jan 4. Eur J Hum Genet. 2017. PMID: 28051070 Free PMC article.
-
Site-Dependent Lineage Preference of Adipose Stem Cells.Front Cell Dev Biol. 2020 Apr 15;8:237. doi: 10.3389/fcell.2020.00237. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32351957 Free PMC article.
-
Blood hsa-miR-122-5p and hsa-miR-885-5p levels associate with fatty liver and related lipoprotein metabolism-The Young Finns Study.Sci Rep. 2016 Dec 5;6:38262. doi: 10.1038/srep38262. Sci Rep. 2016. PMID: 27917915 Free PMC article. Clinical Trial.
References
-
- Olkkonen VM, Lehto M (2004) Oxysterols and oxysterol binding proteins: Role in lipid metabolism and atherosclerosis. Ann Med 36: 562–572. - PubMed
-
- Cowart LA (2009) Sphingolipids: Players in the pathology of metabolic disease. Trends Endocrinol Metab 20: 34–42. - PubMed
-
- Nagao K, Yanagita T (2008) Bioactive lipids in metabolic syndrome. Prog Lipid Res 47: 127–146. - PubMed
-
- Bjorkhem I, Diczfalusy U (2002) Oxysterols: Friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Biol 22: 734–742. - PubMed
-
- Lordan S, O’Brien NM, Mackrill JJ (2009) The role of calcium in apoptosis induced by 7beta-hydroxycholesterol and cholesterol-5beta,6beta-epoxide. J Biochem Mol Toxicol 23: 324–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

