A lentiviral gene therapy strategy for the in vitro production of feline erythropoietin
- PMID: 23028782
- PMCID: PMC3445592
- DOI: 10.1371/journal.pone.0045099
A lentiviral gene therapy strategy for the in vitro production of feline erythropoietin
Abstract
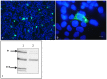
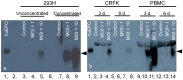
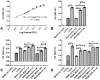
Nonregenerative anemia due to chronic renal failure is a common problem in domestic cats. Unfortunately, administration of recombinant human erythropoietin often only improves anemia temporarily due to antibody development. In this in vitro study, feline erythropoietin cDNA was cloned from feline renal tissue and utilized in the construction of a replication-defective lentiviral vector. The native recombinant feline erythropoietin (rfEPO) sequence was confirmed by sequencing. Upon viral vector infection of human 293H cells, Crandall Renal Feline Kidney cell line and primary feline peripheral blood mononuclear cells, bioactive rfEPO protein was produced. The presence of cellular rfEPO cDNA was confirmed by standard PCR, production of abundant rfEPO mRNA was confirmed by real-time PCR, and secretion of rfEPO protein was demonstrated by Western blot analyses, while rfEPO protein bioactivity was confirmed via an MTT proliferation bioassay. This in vitro study demonstrates the feasibility of a replication-defective lentiviral vector delivery system for the in vitro production of biologically active feline erythropoietin. Anemic cats with chronic renal failure represent a potential in vivo application of a lentiviral gene therapy system.
Conflict of interest statement
Figures

Similar articles
-
Expression, bioactivity, and clinical assessment of recombinant feline erythropoietin.Am J Vet Res. 2004 Oct;65(10):1355-66. doi: 10.2460/ajvr.2004.65.1355. Am J Vet Res. 2004. PMID: 15524322
-
A lentivirus-vectored feline erythropoietin gene therapy strategy in tissue culture and rodent models for the potential treatment of chronic renal disease-associated anemia.Am J Vet Res. 2024 Apr 20;85(6):ajvr.23.12.0280. doi: 10.2460/ajvr.23.12.0280. Print 2024 Jun 1. Am J Vet Res. 2024. PMID: 38626794
-
Transient and stable transfection of Chinese hamster ovary cells with the recombinant feline erythropoietin gene and expression, purification, and biological activity of feline erythropoietin protein.Am J Vet Res. 2003 Dec;64(12):1465-71. doi: 10.2460/ajvr.2003.64.1465. Am J Vet Res. 2003. PMID: 14672421
-
[Construction of lentiviral vector containing Homo sapiens forkhead box C2 gene and its expression in bone marrow mesenchymal stem cells of rabbits].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 May;27(5):535-40. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 23879088 Chinese.
-
Anti-HIV-1 gene expressing lentiviral vectors as an adjunctive therapy for HIV-1 infection.Curr HIV Res. 2004 Apr;2(2):185-91. doi: 10.2174/1570162043484906. Curr HIV Res. 2004. PMID: 15078182 Review.
References
-
- Koury ST, Bondurant MC, Koury MJ (1988) Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood 71: 524–527. - PubMed
-
- Walker MC, Mandell TC, Crawford PC, Simon GG, Cahill KS, et al. (2005) Expression of erythropoietin in cats treated with a recombinant adeno-associated viral vector. Am J Vet Res 66: 450–456. - PubMed
-
- Fisher JW, Koury S, Ducey T, Mendel S (1996) Erythropoietin production by interstitial cells of hypoxic monkey kidneys. Br J Haematol 95: 27–32. - PubMed
-
- Elliott J, Barber PJ (1998) Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 39: 78–85. - PubMed
-
- DiBartola SP, Rutgers HC, Zack PM, Tarr MJ (1987) Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 190: 1196–1202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

