Obese rats exhibit high levels of fat necrosis and isoprostanes in taurocholate-induced acute pancreatitis
- PMID: 23028532
- PMCID: PMC3445528
- DOI: 10.1371/journal.pone.0044383
Obese rats exhibit high levels of fat necrosis and isoprostanes in taurocholate-induced acute pancreatitis
Abstract
Background: Obesity is a prognostic factor for severity in acute pancreatitis in humans. Our aim was to assess the role of oxidative stress and abdominal fat in the increased severity of acute pancreatitis in obese rats.
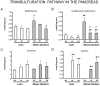
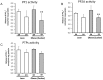
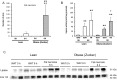
Methodology: Taurocholate-induced acute pancreatitis was performed in lean and obese Zucker rats. Levels of reduced glutathione, oxidized glutathione, L-cysteine, cystine, and S-adenosylmethionine were measured in pancreas as well as the activities of serine/threonine protein phosphatases PP1 and PP2A and tyrosin phosphatases. Isoprostane, malondialdehyde, triglyceride, and free fatty acid levels and lipase activity were measured in plasma and ascites. Lipase activity was measured in white adipose tissue with and without necrosis and confirmed by western blotting.
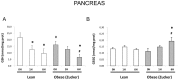
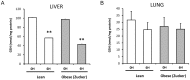
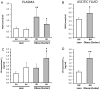
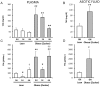
Findings: Under basal conditions obese rats exhibited lower reduced glutathione levels in pancreas and higher triglyceride and free fatty acid levels in plasma than lean rats. S-adenosyl methionine levels were markedly increased in pancreas of obese rats. Acute pancreatitis in obese rats led to glutathione oxidation and lower reduced glutathione levels in pancreas together with decreased activities of redox-sensitive phosphatases PP1, and PP2A. S-adenosyl methionine levels decreased but cystine levels increased markedly in pancreas upon pancreatitis. Acute pancreatitis triggered an increase in isoprostane levels in plasma and ascites in obese rats. Free fatty acid levels were extremely high in pancreatitis-associated ascitic fluid from obese rats and lipase was bound with great affinity to white adipose tissue, especially to areas of necrosis.
Conclusions: Our results show that oxidative stress occurs locally and systemically in obese rats with pancreatitis favouring inactivation of protein phosphatases in pancreas, which would promote up-regulation of pro-inflammatory cytokines, and the increase of isoprostanes which might cause powerful pulmonary and renal vasoconstriction. Future studies are needed to confirm the translational relevance of the present findings obtained in a rat model of taurocholate-induced pancreatic damage and necrosis.
Conflict of interest statement
Figures
Similar articles
-
Pancreatic ascites hemoglobin contributes to the systemic response in acute pancreatitis.Free Radic Biol Med. 2015 Apr;81:145-55. doi: 10.1016/j.freeradbiomed.2014.08.008. Epub 2014 Aug 23. Free Radic Biol Med. 2015. PMID: 25157787
-
Obesity alters cytokine gene expression and promotes liver injury in rats with acute pancreatitis.Obesity (Silver Spring). 2008 Jan;16(1):23-8. doi: 10.1038/oby.2007.27. Obesity (Silver Spring). 2008. PMID: 18223607
-
Obesity causes PGC-1α deficiency in the pancreas leading to marked IL-6 upregulation via NF-κB in acute pancreatitis.J Pathol. 2019 Jan;247(1):48-59. doi: 10.1002/path.5166. Epub 2018 Dec 11. J Pathol. 2019. PMID: 30221360
-
[Protective effect of nitric oxide on pancreas and its relation to sulfhydryl compounds and oxygen free radicals].Zhonghua Wai Ke Za Zhi. 2000 Dec;38(12):928-30. Zhonghua Wai Ke Za Zhi. 2000. PMID: 11832200 Chinese.
-
Role of redox signaling, protein phosphatases and histone acetylation in the inflammatory cascade in acute pancreatitis. Therapeutic implications.Inflamm Allergy Drug Targets. 2010 Jun;9(2):97-108. doi: 10.2174/187152810791292773. Inflamm Allergy Drug Targets. 2010. PMID: 20361855 Review.
Cited by
-
Redox signaling in acute pancreatitis.Redox Biol. 2015 Aug;5:1-14. doi: 10.1016/j.redox.2015.01.014. Epub 2015 Jan 28. Redox Biol. 2015. PMID: 25778551 Free PMC article. Review.
-
The Effect of the Body Mass Indexes of Young Healthy Individuals on the Glyacemic Indexes of Traditional and Modified Vegetarian Meals.Nutrients. 2019 Oct 22;11(10):2546. doi: 10.3390/nu11102546. Nutrients. 2019. PMID: 31652553 Free PMC article.
-
Serum S-adenosylmethionine, but not methionine, increases in response to overfeeding in humans.Nutr Diabetes. 2016 Jan 25;6(1):e192. doi: 10.1038/nutd.2015.44. Nutr Diabetes. 2016. PMID: 26807510 Free PMC article.
-
Effect of Sheng-jiang powder on multiple-organ inflammatory injury in acute pancreatitis in rats fed a high-fat diet.World J Gastroenterol. 2019 Feb 14;25(6):683-695. doi: 10.3748/wjg.v25.i6.683. World J Gastroenterol. 2019. PMID: 30783372 Free PMC article.
-
Eurytrema coelomaticum natural infection in small ruminants: a neglected condition.Parasitology. 2021 Apr;148(5):576-583. doi: 10.1017/S0031182020002358. Epub 2020 Dec 14. Parasitology. 2021. PMID: 33314998 Free PMC article.
References
-
- Ellis MP, French JJ, Charnley RM (2009) Acute pancreatitis and the influence of socioeconomic deprivation. Br J Surg 96: 74–80. - PubMed
-
- Pandol SJ, Saluja AK, Imrie CW, Banks PA (2007) Gastroenterology 133: 1056.e1–e25. - PubMed
-
- Porter KA, Banks PA (1991) Obesity as a predictor of severity in acute pancreatitis. Int J Pancreatol 10: 247–252. - PubMed
-
- Martínez J, Sánchez-Payá J, Palazón JM, Aparicio JR, Picó A, et al. (1999) Obesity: a prognostic factor of severity in acute pancreatitis. Pancreas 19: 15–20. - PubMed
-
- Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC (2006) Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology 6: 279–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

