Citrullination of histone H3 interferes with HP1-mediated transcriptional repression
- PMID: 23028349
- PMCID: PMC3441713
- DOI: 10.1371/journal.pgen.1002934
Citrullination of histone H3 interferes with HP1-mediated transcriptional repression
Abstract
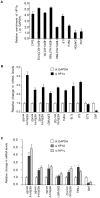
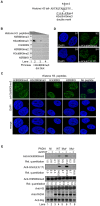
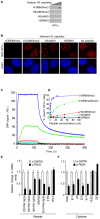
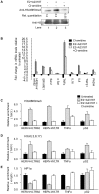
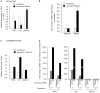
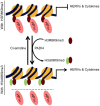
Multiple Sclerosis (MS) is an autoimmune disease associated with abnormal expression of a subset of cytokines, resulting in inappropriate T-lymphocyte activation and uncontrolled immune response. A key issue in the field is the need to understand why these cytokines are transcriptionally activated in the patients. Here, we have examined several transcription units subject to pathological reactivation in MS, including the TNFα and IL8 cytokine genes and also several Human Endogenous RetroViruses (HERVs). We find that both the immune genes and the HERVs require the heterochromatin protein HP1α for their transcriptional repression. We further show that the Peptidylarginine Deiminase 4 (PADI4), an enzyme with a suspected role in MS, weakens the binding of HP1α to tri-methylated histone H3 lysine 9 by citrullinating histone H3 arginine 8. The resulting de-repression of both cytokines and HERVs can be reversed with the PADI-inhibitor Cl-amidine. Finally, we show that in peripheral blood mononuclear cells (PBMCs) from MS patients, the promoters of TNFα, and several HERVs share a deficit in HP1α recruitment and an augmented accumulation of histone H3 with a double citrulline 8 tri-methyl lysine 9 modifications. Thus, our study provides compelling evidence that HP1α and PADI4 are regulators of both immune genes and HERVs, and that multiple events of transcriptional reactivation in MS patients can be explained by the deficiency of a single mechanism of gene silencing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Potential role for PADI-mediated histone citrullination in preimplantation development.BMC Dev Biol. 2012 Jun 19;12:19. doi: 10.1186/1471-213X-12-19. BMC Dev Biol. 2012. PMID: 22712504 Free PMC article.
-
Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation.J Neurosci. 2006 Nov 1;26(44):11387-96. doi: 10.1523/JNEUROSCI.3349-06.2006. J Neurosci. 2006. PMID: 17079667 Free PMC article.
-
Citrullination regulates pluripotency and histone H1 binding to chromatin.Nature. 2014 Mar 6;507(7490):104-8. doi: 10.1038/nature12942. Epub 2014 Jan 26. Nature. 2014. PMID: 24463520 Free PMC article.
-
Role of citrullination modification catalyzed by peptidylarginine deiminase 4 in gene transcriptional regulation.Acta Biochim Biophys Sin (Shanghai). 2017 Jul 1;49(7):567-572. doi: 10.1093/abbs/gmx042. Acta Biochim Biophys Sin (Shanghai). 2017. PMID: 28472221 Review.
-
Peptidylarginine deiminase 4 and citrullination in health and disease.Autoimmun Rev. 2010 Jan;9(3):158-60. doi: 10.1016/j.autrev.2009.06.002. Epub 2009 Jun 18. Autoimmun Rev. 2010. PMID: 19540364 Review.
Cited by
-
PAD4 and Its Inhibitors in Cancer Progression and Prognosis.Pharmaceutics. 2022 Nov 8;14(11):2414. doi: 10.3390/pharmaceutics14112414. Pharmaceutics. 2022. PMID: 36365233 Free PMC article. Review.
-
A comprehensive view of the epigenetic landscape. Part II: Histone post-translational modification, nucleosome level, and chromatin regulation by ncRNAs.Neurotox Res. 2015 Feb;27(2):172-97. doi: 10.1007/s12640-014-9508-6. Epub 2014 Dec 17. Neurotox Res. 2015. PMID: 25516120 Free PMC article. Review.
-
Histone Modifications and Cancer.Cold Spring Harb Perspect Biol. 2016 Apr 1;8(4):a019521. doi: 10.1101/cshperspect.a019521. Cold Spring Harb Perspect Biol. 2016. PMID: 27037415 Free PMC article. Review.
-
Small molecule activates citrullination through targeting PAD2.Philos Trans R Soc Lond B Biol Sci. 2023 Nov 20;378(1890):20220248. doi: 10.1098/rstb.2022.0248. Epub 2023 Oct 2. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37778388 Free PMC article.
-
Chemical biology of protein arginine modifications in epigenetic regulation.Chem Rev. 2015 Jun 10;115(11):5413-61. doi: 10.1021/acs.chemrev.5b00003. Epub 2015 May 13. Chem Rev. 2015. PMID: 25970731 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

