Tollip, an intracellular trafficking protein, is a novel modulator of the transforming growth factor-β signaling pathway
- PMID: 23027871
- PMCID: PMC3501082
- DOI: 10.1074/jbc.M112.388009
Tollip, an intracellular trafficking protein, is a novel modulator of the transforming growth factor-β signaling pathway
Abstract
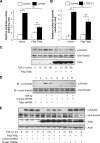
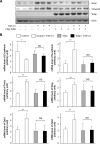
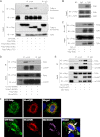
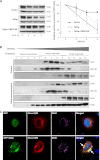
Upon activation, TGF-β type I receptor (TβRI) undergoes active ubiquitination via recruitment of E3 ligases to the receptor complex by Smad7. However, how ubiquitination of TβRI is coupled to intracellular trafficking, and protein degradation remains unclear. We report here that Tollip, an adaptor protein that contains both ubiquitin-associated domains and endosome-targeting domain, plays an important role in modulating trafficking and degradation of TβRI. Tollip was previously demonstrated to possess a functional role in modulating the signaling of interleukin-1 and Toll-like receptors. We identify here that Tollip interacts with Smad7, a major modulatory protein involved in the negative regulation of TGF-β signaling. Overexpression of Tollip antagonizes TGF-β-stimulated transcriptional response, Smad2 phosphorylation, and epithelial-mesenchymal transition. Tollip also interacts with ubiquitinated TβRI, and such interaction requires ubiquitin-associated domains of Tollip. The interaction and intracellular colocalization of Tollip with TβRI is enhanced by Smad7. Overexpression of Tollip accelerates protein degradation of activated TβRI. In addition, Tollip alters subcellular compartmentalization and endosomal trafficking of activated TβRI. Collectively, our studies reveal that Tollip cooperates with Smad7 to modulate intracellular trafficking and degradation of ubiquitinated TβRI, whereby negatively regulates TGF-β signaling pathway.
Figures



Similar articles
-
TSC-22 promotes transforming growth factor β-mediated cardiac myofibroblast differentiation by antagonizing Smad7 activity.Mol Cell Biol. 2011 Sep;31(18):3700-9. doi: 10.1128/MCB.05448-11. Epub 2011 Jul 26. Mol Cell Biol. 2011. PMID: 21791611 Free PMC article.
-
VprBP mitigates TGF-β and Activin signaling by promoting Smurf1-mediated type I receptor degradation.J Mol Cell Biol. 2020 Feb 20;12(2):138-151. doi: 10.1093/jmcb/mjz057. J Mol Cell Biol. 2020. PMID: 31291647 Free PMC article.
-
NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor.Biochem J. 2005 Mar 15;386(Pt 3):461-70. doi: 10.1042/BJ20040738. Biochem J. 2005. PMID: 15496141 Free PMC article.
-
Inhibitory Smad7: emerging roles in health and disease.Curr Mol Pharmacol. 2011 Jun;4(2):141-53. Curr Mol Pharmacol. 2011. PMID: 21222648 Review.
-
Current perspectives on inhibitory SMAD7 in health and disease.Crit Rev Biochem Mol Biol. 2020 Dec;55(6):691-715. doi: 10.1080/10409238.2020.1828260. Epub 2020 Oct 20. Crit Rev Biochem Mol Biol. 2020. PMID: 33081543 Review.
Cited by
-
TOLLIP Protein Expression Predicts Unfavorable Outcome in Renal Cell Carcinoma.Int J Mol Sci. 2022 Nov 25;23(23):14702. doi: 10.3390/ijms232314702. Int J Mol Sci. 2022. PMID: 36499030 Free PMC article.
-
The multifunctional adaptor protein HIP-55 couples Smad7 to accelerate TGF-β type I receptor degradation.Acta Pharmacol Sin. 2022 Mar;43(3):634-644. doi: 10.1038/s41401-021-00741-1. Epub 2021 Jul 30. Acta Pharmacol Sin. 2022. PMID: 34331017 Free PMC article.
-
Tollip Deficiency Alters Atherosclerosis and Steatosis by Disrupting Lipophagy.J Am Heart Assoc. 2017 Apr 10;6(4):e004078. doi: 10.1161/JAHA.116.004078. J Am Heart Assoc. 2017. PMID: 28396568 Free PMC article.
-
CIN85 modulates TGFβ signaling by promoting the presentation of TGFβ receptors on the cell surface.J Cell Biol. 2015 Jul 20;210(2):319-32. doi: 10.1083/jcb.201411025. Epub 2015 Jul 13. J Cell Biol. 2015. PMID: 26169354 Free PMC article.
-
Roles of Myosin-Mediated Membrane Trafficking in TGF-β Signaling.Int J Mol Sci. 2019 Aug 12;20(16):3913. doi: 10.3390/ijms20163913. Int J Mol Sci. 2019. PMID: 31408934 Free PMC article. Review.
References
-
- Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem. 67, 753–791 - PubMed
-
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. (1992) TGF β signals through a heteromeric protein kinase receptor complex. Cell 71, 1003–1014 - PubMed
-
- Wrana J. L., Attisano L. (2000) The Smad pathway. Cytokine Growth Factor Rev. 11, 5–13 - PubMed
-
- Nakao A., Afrakhte M., Morén A., Nakayama T., Christian J. L., Heuchel R., Itoh S., Kawabata M., Heldin N. E., Heldin C. H., ten Dijke P. (1997) Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 389, 631–635 - PubMed
-
- Kavsak P., Rasmussen R. K., Causing C. G., Bonni S., Zhu H., Thomsen G. H., Wrana J. L. (2000) Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF β receptor for degradation. Mol. Cell 6, 1365–1375 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

